Hyperconjugation-mediated solvent effects in phosphoanhydride bonds
- PMID: 23009395
- PMCID: PMC3491982
- DOI: 10.1021/jp306607k
Hyperconjugation-mediated solvent effects in phosphoanhydride bonds
Abstract
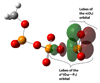
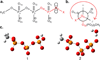
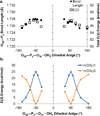
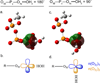
Density functional theory and natural bond orbital analysis are used to explore the impact of solvent on hyperconjugation in methyl triphosphate, a model for "energy rich" phosphoanhydride bonds, such as found in ATP. As expected, dihedral rotation of a hydroxyl group vicinal to the phosphoanhydride bond reveals that the conformational dependence of the anomeric effect involves modulation of the orbital overlap between the donor and acceptor orbitals. However, a conformational independence was observed in the rotation of a solvent hydrogen bond. As one lone pair orbital rotates away from an optimal antiperiplanar orientation, the overall magnitude of the anomeric effect is compensated approximately by the other lone pair as it becomes more antiperiplanar. Furthermore, solvent modulation of the anomeric effect is not restricted to the antiperiplanar lone pair; hydrogen bonds involving gauche lone pairs also affect the anomeric interaction and the strength of the phosphoanhydride bond. Both gauche and anti solvent hydrogen bonds lengthen nonbridging O-P bonds, increasing the distance between donor and acceptor orbitals and decreasing orbital overlap, which leads to a reduction of the anomeric effect. Solvent effects are additive with greater reduction in the anomeric effect upon increasing water coordination. By controlling the coordination environment of substrates in an active site, kinases, phosphatases, and other enzymes important in metabolism and signaling may have the potential to modulate the stability of individual phosphoanhydride bonds through stereoelectronic effects.
Figures





Similar articles
-
Effect of Orbital Interactions between Vicinal Bonds and between Hydroxy Groups on the Conformational Stabilities of 1,2-Ethanediol and 2,3-Butanediols.J Phys Chem A. 2017 Nov 9;121(44):8484-8494. doi: 10.1021/acs.jpca.7b08085. Epub 2017 Oct 27. J Phys Chem A. 2017. PMID: 29028337
-
Anomeric effect in "high energy" phosphate bonds. Selective destabilization of the scissile bond and modulation of the exothermicity of hydrolysis.J Am Chem Soc. 2008 Mar 19;130(11):3349-58. doi: 10.1021/ja073652x. Epub 2008 Feb 27. J Am Chem Soc. 2008. PMID: 18302368
-
Hydrogen bonding mediated by key orbital interactions determines hydration enthalpy differences of phosphate water clusters.J Phys Chem A. 2007 Oct 25;111(42):10804-14. doi: 10.1021/jp0748112. Epub 2007 Oct 4. J Phys Chem A. 2007. PMID: 17915844
-
Stereoelectronic power of oxygen in control of chemical reactivity: the anomeric effect is not alone.Chem Soc Rev. 2021 Sep 20;50(18):10253-10345. doi: 10.1039/d1cs00386k. Chem Soc Rev. 2021. PMID: 34263287 Review.
-
Anomeric effect, hyperconjugation and electrostatics: lessons from complexity in a classic stereoelectronic phenomenon.Chem Soc Rev. 2021 Sep 20;50(18):10212-10252. doi: 10.1039/d1cs00564b. Chem Soc Rev. 2021. PMID: 34542133 Review.
Cited by
-
Common hydrogen bond interactions in diverse phosphoryl transfer active sites.PLoS One. 2014 Sep 19;9(9):e108310. doi: 10.1371/journal.pone.0108310. eCollection 2014. PLoS One. 2014. PMID: 25238155 Free PMC article.
-
Structure of McsB, a protein kinase for regulated arginine phosphorylation.Nat Chem Biol. 2019 May;15(5):510-518. doi: 10.1038/s41589-019-0265-y. Epub 2019 Apr 8. Nat Chem Biol. 2019. PMID: 30962626 Free PMC article.
References
-
- Berg J, Tymoczko JL, Stryer L. Biochemistry. 5th ed. New York: W.H. Freeman & Co.; 2002.
-
- Siebold C, Arnold I, Garcia-Alles LF, Baumann U, Erni B. J. Biol. Chem. 2003;278:48236. - PubMed
-
- Srivastava SK, Rajasree K, Gopal B. Biochim. Biophys. Acta. 2011;1814:1349. - PubMed
-
- Yousef MS, Fabiola F, Gattis JL, Somasundaram T, Chapman MS. Acta Crystallogr. D Biol. Crystallogr. 2002;58:2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

