Na(+)/K(+)-ATPase expression changes in the rabbit lacrimal glands during pregnancy
- PMID: 23009595
- PMCID: PMC3636992
- DOI: 10.3109/02713683.2012.725797
Na(+)/K(+)-ATPase expression changes in the rabbit lacrimal glands during pregnancy
Abstract
Purpose: To investigate the expressional changes of Na(+)/K(+)-ATPase subunits in the lacrimal glands (LG) of term pregnant rabbits.
Methods: LG were obtained from term pregnant rabbits and age-matched female control rabbits for laser capture microdissection (LCM), real time RT-PCR, western blot, and immunofluorescence. The mRNA and proteins of α1, α2, β1, β2, and β3 subunits of Na(+)/K(+)-ATPase were detected and quantified.
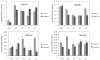
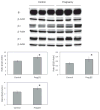
Results: Although only the mRNA for β3 from whole LG of pregnant rabbits was significantly different from that of normal controls, many mRNA levels for α1, α2, β1, β2, and β3 from acini and epithelial cells from various duct segments that were collected by LCM were significantly different from those of normal control rabbits. Western blots demonstrated that the expressions of all three β subunits were significantly higher in pregnant rabbits, while both α subunits remained unchanged during pregnancy. Interestingly, immunofluorescence results showed that the distribution patterns of all Na(+)/K(+)-ATPase subunits during pregnancy were similar to those of the control rabbits.
Conclusions: Changes were found in mRNA and protein expressions of Na(+)/K(+)-ATPase subunits in LG from term pregnant rabbits and these changes suggest a role in the pregnancy-related LG secretion changes and dry eye symptoms observed in these animals.
Conflict of interest statement
Figures






Similar articles
-
Na(+)/K(+)-ATPase in the lacrimal glands of rabbits and its changes during induced autoimmune dacryoadenitis.Mol Vis. 2011;17:2368-79. Epub 2011 Sep 1. Mol Vis. 2011. PMID: 21921989 Free PMC article.
-
Changes of the ocular surface and aquaporins in the lacrimal glands of rabbits during pregnancy.Mol Vis. 2011;17:2847-55. Epub 2011 Nov 9. Mol Vis. 2011. PMID: 22128232 Free PMC article.
-
ENaC in the Rabbit Lacrimal Gland and its Changes During Sjögren Syndrome and Pregnancy.Eye Contact Lens. 2015 Sep;41(5):297-303. doi: 10.1097/ICL.0000000000000123. Eye Contact Lens. 2015. PMID: 25828511 Free PMC article.
-
Changes of aquaporins in the lacrimal glands of a rabbit model of Sjögren's syndrome.Curr Eye Res. 2011 Jun;36(6):571-8. doi: 10.3109/02713683.2011.574330. Epub 2011 Apr 27. Curr Eye Res. 2011. PMID: 21524183 Free PMC article.
-
Aquaporins in lacrimal glands and their role in dry eye disease.Exp Eye Res. 2023 Nov;236:109676. doi: 10.1016/j.exer.2023.109676. Epub 2023 Oct 10. Exp Eye Res. 2023. PMID: 37827442 Review.
Cited by
-
Inflammation mechanism and anti-inflammatory therapy of dry eye.Front Med (Lausanne). 2024 Feb 14;11:1307682. doi: 10.3389/fmed.2024.1307682. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38420354 Free PMC article. Review.
-
Should I Get LASIK If I'm Breastfeeding?Ophthalmol Ther. 2019 Sep;8(3):349-352. doi: 10.1007/s40123-019-0195-5. Epub 2019 Jun 28. Ophthalmol Ther. 2019. PMID: 31254257 Free PMC article.
-
Ion channels in dry eye disease.Indian J Ophthalmol. 2023 Apr;71(4):1215-1226. doi: 10.4103/IJO.IJO_3020_22. Indian J Ophthalmol. 2023. PMID: 37026252 Free PMC article. Review.
References
-
- Mircheff AK. Lacrimal fluid and electrolyte secretion: a review. Curr Eye Res. 1989;8:607–617. - PubMed
-
- Herok GH, Millar TJ, Anderton PJ, Martin DK. Characterization of an inwardly rectifying potassium channel in the rabbit superior lacrimal gland. Invest Ophthalmol Vis Sci. 1998;39:308–314. - PubMed
-
- Herok GH, Millar TJ, Anderton PJ, Martin DK. Role of chloride channels in regulating the volume of acinar cells of the rabbit superior lacrimal gland. Invest Ophthalmol Vis Sci. 2008;49:5517–5525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
