Monocenter feasibility study of the MRI compatibility of the Evia pacemaker in combination with Safio S pacemaker lead
- PMID: 23009683
- PMCID: PMC3482396
- DOI: 10.1186/1532-429X-14-67
Monocenter feasibility study of the MRI compatibility of the Evia pacemaker in combination with Safio S pacemaker lead
Abstract
Background: The purpose of this study was to evaluate the feasibility of the magnetic resonance (MR) conditional pacemaker (PM) system (Evia SR-T and DR-T with Safio S leads) under MR conditions.
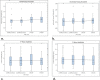
Methods: Patients with standard PM indications and Evia PM were eligible for enrollment in this single center prospective non-randomized pilot study. Patients underwent MR of the brain and lower lumbar spine at 1.5 Tesla. Atrial (RA) und ventricular (RV) lead parameters (sensing, pacing threshold [PTH], pacing impedance) were assessed immediately before (baseline follow-up [FU]) and immediately after MRI (1st FU), after 1 month (2nd FU) and 3 months (3rd FU). The effect of MR on serious adverse device effect (SADE) free-rate, on atrial and ventricular sensing (AS/VS; mV) and atrial (RA) and ventricular (RV) pacing thresholds (PTH; V/0.4 ms) were investigated between baseline and 2nd FU. Continuous variables are expressed as mean ± SD and were compared using paired Student's t-test. A p < 0.05 was considered significant.
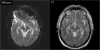
Results: Thirty-one patients were enrolled. One patient had to be excluded because of an enrollment violation. Therefore, data of 30 patients (female 12 [40%], age 73 ± 12 years, dual chamber PM 15 [50%]) were included in this analysis. No MR related SADE occurred. Lead measurements were not statistically different between the baseline FU and the 2nd FU (AS/VS at baseline 3.2 ± 2.1/15.0 ± 6.0, at 2nd FU 3.2 ± 2.1/14.9 ± 6.5; p = ns. RA-PTH/RV-PTH at baseline 0.68 ± 0.18/0.78 ± 0.22, at 2nd FU 0.71 ± 0.24/0.78 ± 0.22; p = ns). The presence of the permanent pacemakers led to MR imaging artifacts on diffusion weighted sequences of the brain, but did not affect other sequences (e.g. FLAIR and T2 weighted spin-echo images).
Conclusion: The use of the MR conditional Evia PM in a MR environment under predefined conditions is feasible. No MR related SADEs nor clinically relevant changes in device functions occurred.
Figures

Similar articles
-
Safe performance of magnetic resonance of the heart in patients with magnetic resonance conditional pacemaker systems: the safety issue of the ESTIMATE study.J Cardiovasc Magn Reson. 2014 May 6;16(1):30. doi: 10.1186/1532-429X-16-30. J Cardiovasc Magn Reson. 2014. PMID: 24886167 Free PMC article.
-
A detailed view on pacemaker lead parameters remotely transmitted after magnetic resonance.Pacing Clin Electrophysiol. 2015 Jun;38(6):746-57. doi: 10.1111/pace.12628. Epub 2015 Apr 16. Pacing Clin Electrophysiol. 2015. PMID: 25787901
-
Clinical safety of the ProMRI pacemaker system in patients subjected to head and lower lumbar 1.5-T magnetic resonance imaging scanning conditions.Heart Rhythm. 2015 Jun;12(6):1183-91. doi: 10.1016/j.hrthm.2015.02.010. Epub 2015 Feb 11. Heart Rhythm. 2015. PMID: 25680307
-
Cardiovascular magnetic resonance in patients with magnetic resonance conditional pacemaker systems at 1.5 T: influence of pacemaker related artifacts on image quality including first pass perfusion, aortic and mitral valve assessment, flow measurement, short tau inversion recovery and T1-weighted imaging.Int J Cardiovasc Imaging. 2017 Mar;33(3):383-394. doi: 10.1007/s10554-016-1012-z. Epub 2016 Nov 4. Int J Cardiovasc Imaging. 2017. PMID: 27815793 Review.
-
Magnetic resonance imaging in patients with temporary external pacemakers.Europace. 2022 Dec 9;24(12):1960-1966. doi: 10.1093/europace/euac147. Europace. 2022. PMID: 36006800 Review.
Cited by
-
Review of Journal of Cardiovascular Magnetic Resonance 2013.J Cardiovasc Magn Reson. 2014 Dec 5;16:100. doi: 10.1186/s12968-014-0100-2. J Cardiovasc Magn Reson. 2014. PMID: 25475898 Free PMC article. Review.
-
Clinical safety of ProMRI implantable cardioverter-defibrillator systems during head and lower lumbar magnetic resonance imaging at 1.5 Tesla.Sci Rep. 2019 Dec 3;9(1):18243. doi: 10.1038/s41598-019-54342-4. Sci Rep. 2019. PMID: 31796767 Free PMC article.
-
MRI-conditional pacemakers: current perspectives.Med Devices (Auckl). 2014 May 7;7:115-24. doi: 10.2147/MDER.S44063. eCollection 2014. Med Devices (Auckl). 2014. PMID: 24851058 Free PMC article. Review.
-
Magnetic resonance imaging in patients with a subcutaneous implantable cardioverter-defibrillator.Europace. 2015 May;17(5):761-6. doi: 10.1093/europace/euu377. Epub 2015 Feb 16. Europace. 2015. PMID: 25687749 Free PMC article.
-
Clinical safety of the ProMRI implantable cardioverter-defibrillator systems during head and lower lumbar magnetic resonance imaging at 3 T: results of the ProMRI 3T ENHANCED Master study.Europace. 2019 Nov 1;21(11):1678-1685. doi: 10.1093/europace/euz189. Europace. 2019. PMID: 31322701 Free PMC article.
References
-
- American College of Radiology. ACR Practice Guidelines. http://www.acr.org.
-
- Sommer T, Naehle CP, Yang A, Zeijlemaker V, Hackenbroch M, Schmiedel A, Meyer C, Strach K, Skowasch D, Vahlhaus C, Litt H, Schild H. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 Tesla in the presence of cardiac pacemakers in non-pacemaker dependent patients. A prospective study with 115 examinations. Circulation. 2006;114:1285–1292. doi: 10.1161/CIRCULATIONAHA.105.597013. - DOI - PubMed
-
- Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, Weiss RG, Berger RD, Bluemke DA, Halperin HR. Clinicial utility of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 Tesla. Circulation. 2006;114:1277–1284. doi: 10.1161/CIRCULATIONAHA.105.607655. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

