Engineered measles virus Edmonston strain used as a novel oncolytic viral system against human hepatoblastoma
- PMID: 23009685
- PMCID: PMC3488522
- DOI: 10.1186/1471-2407-12-427
Engineered measles virus Edmonston strain used as a novel oncolytic viral system against human hepatoblastoma
Abstract
Background: Hepatoblastoma (HB) is the most common primary, malignant pediatric liver tumor in children. The treatment results for affected children have markedly improved in recent decades. However, the prognosis for high-risk patients who have extrahepatic extensions, invasion of the large hepatic veins, distant metastases and very high alpha-fetoprotein (AFP) serum levels remains poor. There is an urgent need for the development of novel therapeutic approaches.
Methods: An attenuated strain of measles virus, derived from the Edmonston vaccine lineage, was genetically engineered to produce carcinoembryonic antigen (CEA). We investigated the antitumor potential of this novel viral agent against human HB both in vitro and in vivo.
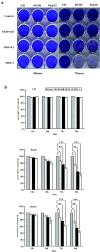
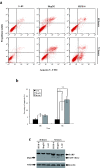
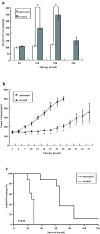
Results: Infection of the Hep2G and HUH6 HB cell lines, at multiplicities of infection (MOIs) ranging from 0.01 to 1, resulted in a significant cytopathic effect consisting of extensive syncytia formation and massive cell death at 72-96 h after infection. Both of the HB lines overexpressed the measles virus receptor CD46 and supported robust viral replication, which correlated with CEA production. The efficacy of this approach in vivo was examined in murine Hep2G xenograft models. Flow cytometry assays indicated an apoptotic mechanism of cell death. Intratumoral administration of MV-CEA resulted in statistically significant delay of tumor growth and prolongation of survival.
Conclusions: The engineered measles virus Edmonston strain MV-CEA has potent therapeutic efficacy against HB cell lines and xenografts. Trackable measles virus derivatives merit further exploration in HB treatment.
Figures
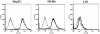
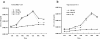
Similar articles
-
A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer.Breast Cancer Res Treat. 2006 Sep;99(2):177-84. doi: 10.1007/s10549-006-9200-5. Epub 2006 Apr 27. Breast Cancer Res Treat. 2006. PMID: 16642271
-
Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme.Cancer Res. 2003 May 15;63(10):2462-9. Cancer Res. 2003. PMID: 12750267
-
Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma.Hepatology. 2006 Dec;44(6):1465-77. doi: 10.1002/hep.21437. Hepatology. 2006. PMID: 17133484
-
Measles virus for cancer therapy.Curr Top Microbiol Immunol. 2009;330:213-41. doi: 10.1007/978-3-540-70617-5_11. Curr Top Microbiol Immunol. 2009. PMID: 19203112 Free PMC article. Review.
-
Oncolytic measles viruses for cancer therapy.Expert Opin Biol Ther. 2004 Oct;4(10):1685-92. doi: 10.1517/14712598.4.10.1685. Expert Opin Biol Ther. 2004. PMID: 15461580 Review.
Cited by
-
Potential and clinical translation of oncolytic measles viruses.Expert Opin Biol Ther. 2017 Mar;17(3):353-363. doi: 10.1080/14712598.2017.1288713. Expert Opin Biol Ther. 2017. PMID: 28129716 Free PMC article. Review.
-
Development and application of oncolytic viruses as the nemesis of tumor cells.Front Microbiol. 2023 Jun 12;14:1188526. doi: 10.3389/fmicb.2023.1188526. eCollection 2023. Front Microbiol. 2023. PMID: 37440883 Free PMC article. Review.
-
Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of pediatric solid tumors.PLoS One. 2014 Jan 30;9(1):e86843. doi: 10.1371/journal.pone.0086843. eCollection 2014. PLoS One. 2014. PMID: 24497984 Free PMC article.
-
A novel, polymer-coated oncolytic measles virus overcomes immune suppression and induces robust antitumor activity.Mol Ther Oncolytics. 2016 Nov 2;3:16022. doi: 10.1038/mto.2016.22. eCollection 2016. Mol Ther Oncolytics. 2016. PMID: 27847861 Free PMC article.
-
Measles Vaccine.Viral Immunol. 2018 Mar;31(2):86-95. doi: 10.1089/vim.2017.0143. Epub 2017 Dec 19. Viral Immunol. 2018. PMID: 29256824 Free PMC article. Review.
References
-
- Häberle B, Bode U, von Schweinitz D. Differentiated treatment protocols for high- and standard-risk hepatoblastoma–an interim report of the German Liver Tumor Study HB99. Klin Padiatr. 2003;215(3):159–165. - PubMed
-
- Zsíros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P. et al.Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28(15):2584–2590. doi: 10.1200/JCO.2009.22.4857. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

