The effect of infection order of porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus on dually infected swine alveolar macrophages
- PMID: 23009687
- PMCID: PMC3528418
- DOI: 10.1186/1746-6148-8-174
The effect of infection order of porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus on dually infected swine alveolar macrophages
Abstract
Background: Concurrent infection with porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) is known as one of the major causes for porcine respiratory disease complex (PRDC). Dual infection with PCV2 and PRRSV is consistently to have more severe clinical presentations and pulmonary lesions than infection with PCV2 alone or PRRSV alone. However, it is not known if dual infections with PCV2 and PRRSV in different infection order may lead to different clinical symptoms in the host. To mimic the possible field conditions, swine alveolar macrophages (AMs) were inoculated with PCV2 and PRRSV in vitro simultaneously or with one virus 18 h earlier than the other. The cell viability, cytopathic effects, antigen-containing rates, phagocytotic and microbial killing capabilities, cytokine profiles (IL-8, TNF-α, and IFN-α) and FasL transcripts were determined, analyzed, and compared to prove the hypothesis.
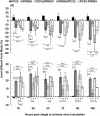
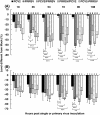
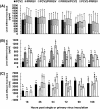
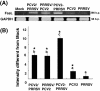
Results: A marked reduction in PRRSV antigen-containing rate, cytopathic effect, and TNF-α expression level was revealed in AMs inoculated with PCV2 and PRRSV simultaneously and in AMs inoculated with PCV2 first then PRRSV 18 h later, but not in AMs inoculated with PRRSV first then PCV2 18 h later. Transient decrease in phagocytosis but constant reduction in microbicidal capability in AMs in the group inoculated with PCV2 alone and constant decrease in phagocytosis and microbicidal capability in AMs in all PRRSV-inoculated groups were noted. The levels of IL-8, TNF-α, IFN-α, and FasL transcripts in AMs in all groups with dual inoculation of PCV2 and PRRSV were significantly increased regardless of the infection orders as compared with infection by PCV2 alone or PRRSV alone.
Conclusions: Swine AMs infected with PCV2 first then PRRSV later or infected with PCV2 and PRRSV simultaneously displayed marked reduction in PRRSV antigen-containing rate, cytopathic effect, and TNF-α expression level. The different inoculation orders of PCV2 and PRRSV in AMs leading to different results in viral antigen positivity, cytopathology, and cytokine profile may explain, at least partially, the underlying mechanism of the enhanced pulmonary lesions in PRDC exerted by dual infection with PCV2 and PRRSV and the variable clinical manifestations of PRDC-affected pigs in the field.
Figures
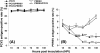
Similar articles
-
Immune Molecules' mRNA Expression in Porcine Alveolar Macrophages Co-Infected with Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2.Viruses. 2023 Mar 17;15(3):777. doi: 10.3390/v15030777. Viruses. 2023. PMID: 36992486 Free PMC article.
-
Reduction of porcine reproductive and respiratory syndrome virus (PRRSV) infection in swine alveolar macrophages by porcine circovirus 2 (PCV2)-induced interferon-alpha.Vet Microbiol. 2005 Jul 1;108(3-4):167-77. doi: 10.1016/j.vetmic.2005.03.010. Vet Microbiol. 2005. PMID: 15936905 Free PMC article.
-
Synergistic effects of sequential infection with highly pathogenic porcine reproductive and respiratory syndrome virus and porcine circovirus type 2.Virol J. 2013 Aug 26;10:265. doi: 10.1186/1743-422X-10-265. Virol J. 2013. PMID: 23971711 Free PMC article.
-
Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae.Vet J. 2016 Jun;212:1-6. doi: 10.1016/j.tvjl.2015.10.030. Epub 2015 Oct 23. Vet J. 2016. PMID: 27256017 Review.
-
The combination of PRRS virus and bacterial endotoxin as a model for multifactorial respiratory disease in pigs.Vet Immunol Immunopathol. 2004 Dec 8;102(3):165-78. doi: 10.1016/j.vetimm.2004.09.006. Vet Immunol Immunopathol. 2004. PMID: 15507303 Free PMC article. Review.
Cited by
-
Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination.Cell Death Differ. 2017 Apr;24(4):683-693. doi: 10.1038/cdd.2017.1. Epub 2017 Feb 3. Cell Death Differ. 2017. PMID: 28157209 Free PMC article.
-
Transcriptional Analysis of PRRSV-Infected Porcine Dendritic Cell Response to Streptococcus suis Infection Reveals Up-Regulation of Inflammatory-Related Genes Expression.PLoS One. 2016 May 23;11(5):e0156019. doi: 10.1371/journal.pone.0156019. eCollection 2016. PLoS One. 2016. PMID: 27213692 Free PMC article.
-
Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type 2.Sci Rep. 2016 Apr 15;6:24401. doi: 10.1038/srep24401. Sci Rep. 2016. PMID: 27080155 Free PMC article.
-
Immune Molecules' mRNA Expression in Porcine Alveolar Macrophages Co-Infected with Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2.Viruses. 2023 Mar 17;15(3):777. doi: 10.3390/v15030777. Viruses. 2023. PMID: 36992486 Free PMC article.
-
Dynamic changes in bronchoalveolar macrophages and cytokines during infection of pigs with a highly or low pathogenic genotype 1 PRRSV strain.Vet Res. 2017 Feb 27;48(1):15. doi: 10.1186/s13567-017-0420-y. Vet Res. 2017. PMID: 28241868 Free PMC article.
References
-
- Brockmeier SL, Halbur PG, Thacker EL. In: Polymicrobial Diseases. Brogden KA, Guthmiller JM, editor. Washington DC: ASM Press; 2002. Porcine respiratory disease complex; pp. 231–258.
-
- Nakharuthai C, Boonsoongnern A, Poolperm P, Wajjwalku W, Urairong K, Chumsing W, Lertwitcharasarakul P, Lekcharoensuk P. Occurrence of swine influenza virus infection in swine with porcine respiratory disease complex. Southeast Asian J Trop Med Public Health. 2008;39(6):1045–1053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

