Expression of Tax-interacting protein 1 (TIP-1) facilitates angiogenesis and tumor formation of human glioblastoma cells in nude mice
- PMID: 23010083
- PMCID: PMC3494810
- DOI: 10.1016/j.canlet.2012.09.011
Expression of Tax-interacting protein 1 (TIP-1) facilitates angiogenesis and tumor formation of human glioblastoma cells in nude mice
Abstract
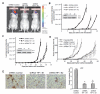
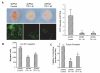
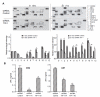
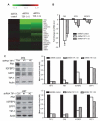
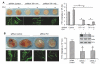
Glioblastoma is the most common and fatal type of primary brain tumors featured with hyperplastic blood vessels. Here, we performed meta-analyses of published data and established a correlation between high TIP-1 expression levels and the poor prognosis of glioblastoma patients. Next, we explored the biological relevance of TIP-1 expression in the pathogenesis of glioblastoma. By using orthotopic and heterotopic mouse models of human glioblastomas, this study has characterized TIP-1 as one contributing factor to the tumor-driven angiogenesis. In vitro and in vivo functional assays, along with biochemical analyses with microarrays and antibody arrays, have demonstrated that TIP-1 utilizes multiple pathways including modulating fibronectin gene expression and uPA protein secretion, to establish or maintain a pro-angiogenic microenvironment within human glioblastoma. In conclusion, this work supports one hypothesis that TIP-1 represents a novel prognostic biomarker and a therapeutic target of human glioblastoma.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures

Similar articles
-
Tax-interacting protein 1 coordinates the spatiotemporal activation of Rho GTPases and regulates the infiltrative growth of human glioblastoma.Oncogene. 2014 Mar 20;33(12):1558-69. doi: 10.1038/onc.2013.97. Epub 2013 Apr 8. Oncogene. 2014. PMID: 23563176 Free PMC article.
-
Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma.Oncol Rep. 2010 Mar;23(3):725-32. Oncol Rep. 2010. PMID: 20127012
-
Involvement of Akt/NF-κB pathway in antitumor effects of parthenolide on glioblastoma cells in vitro and in vivo.BMC Cancer. 2012 Oct 5;12:453. doi: 10.1186/1471-2407-12-453. BMC Cancer. 2012. PMID: 23039130 Free PMC article.
-
Recent advance in molecular angiogenesis in glioblastoma: the challenge and hope for anti-angiogenic therapy.Brain Tumor Pathol. 2015 Oct;32(4):229-36. doi: 10.1007/s10014-015-0233-5. Epub 2015 Oct 5. Brain Tumor Pathol. 2015. PMID: 26437643 Review.
-
Vascular heterogeneity and targeting: the role of YKL-40 in glioblastoma vascularization.Oncotarget. 2015 Dec 1;6(38):40507-18. doi: 10.18632/oncotarget.5943. Oncotarget. 2015. PMID: 26439689 Free PMC article. Review.
Cited by
-
Tax-interacting protein 1 coordinates the spatiotemporal activation of Rho GTPases and regulates the infiltrative growth of human glioblastoma.Oncogene. 2014 Mar 20;33(12):1558-69. doi: 10.1038/onc.2013.97. Epub 2013 Apr 8. Oncogene. 2014. PMID: 23563176 Free PMC article.
-
In vivo immunotherapy of lung cancer using cross-species reactive vascular endothelial growth factor nanobodies.Iran J Basic Med Sci. 2017 May;20(5):489-496. doi: 10.22038/IJBMS.2017.8672. Iran J Basic Med Sci. 2017. PMID: 28656083 Free PMC article.
-
Tax1 binding protein 3 regulates osteogenic and adipogenic differentiation through inactivating Wnt/β-catenin signalling.J Cell Mol Med. 2023 Apr;27(7):950-961. doi: 10.1111/jcmm.17702. Epub 2023 Mar 9. J Cell Mol Med. 2023. PMID: 36892460 Free PMC article.
-
Identification of Prognostic Biomarkers for Glioblastoma Based on Transcriptome and Proteome Association Analysis.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338211035270. doi: 10.1177/15330338211035270. Technol Cancer Res Treat. 2022. PMID: 35538679 Free PMC article.
-
Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW.Br J Neurosurg. 2015;29(6):850-8. doi: 10.3109/02688697.2015.1056090. Epub 2015 Jun 15. Br J Neurosurg. 2015. PMID: 26073144 Free PMC article.
References
-
- Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, Yu IL, Carro MS, Dai F, Tagge MJ, Ferrarese R, Bredel C, Phillips HS, Lukac PJ, Robe PA, Weyerbrock A, Vogel H, Dubner S, Mobley B, He X, Scheck AC, Sikic BI, Aldape KD, Chakravarti A, Harsh G.R.t. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–637. - PMC - PubMed
-
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr., Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science (New York, N.Y. 2008;321:1807–1812. - PMC - PubMed
-
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

