17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm
- PMID: 23010094
- PMCID: PMC3484249
- DOI: 10.1016/j.ajog.2012.09.013
17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm
Abstract
Objective: We sought to evaluate whether 17 alpha-hydroxyprogesterone caproate (17-OHP) reduces preterm birth (PTB) in nulliparous women with a midtrimester cervical length (CL) <30 mm.
Study design: In this multicenter randomized controlled trial, nulliparous women with a singleton gestation between 16 and 22 3/7 weeks with an endovaginal CL <30 mm (<10th percentile in this population) were randomized to weekly intramuscular 17-OHP (250 mg) or placebo through 36 weeks. The primary outcome was PTB <37 weeks.
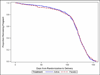
Results: The frequency of PTB did not differ between the 17-OHP (n = 327) and placebo (n = 330) groups (25.1% vs 24.2%; relative risk, 1.03; 95% confidence interval, 0.79-1.35). There also was no difference in the composite adverse neonatal outcome (7.0% vs 9.1%; relative risk, 0.77; 95% confidence interval, 0.46-1.30).
Conclusion: Weekly 17-OHP does not reduce the frequency of PTB in nulliparous women with a midtrimester CL <30 mm.
Trial registration: ClinicalTrials.gov NCT00439374.
Copyright © 2012 Mosby, Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE: The authors report no conflict of interest
Figures
Comment in
-
Progesterone for preterm labour.BJOG. 2016 Nov;123(12):2000. doi: 10.1111/1471-0528.13976. Epub 2016 Mar 30. BJOG. 2016. PMID: 27028883 No abstract available.
References
-
- Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. - PubMed
-
- Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89:417–421. - PubMed
-
- DaFonseca EB, Bittar RE, Carvalho MHB, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. - PubMed
-
- Meis PJ, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. - PubMed
-
- Petrini JR, Callaghan WM, Klebanoff M, et al. Estimated effect of 17 alphahydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U10 HD053118/HD/NICHD NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD53118/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- M01 RR00080/RR/NCRR NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- TL1 RR024147/RR/NCRR NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- UL1 RR024148/RR/NCRR NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- HD53097/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- 5UL1RR025764/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical