A Paracoccidioides brasiliensis glycan shares serologic and functional properties with cryptococcal glucuronoxylomannan
- PMID: 23010152
- PMCID: PMC4290022
- DOI: 10.1016/j.fgb.2012.09.002
A Paracoccidioides brasiliensis glycan shares serologic and functional properties with cryptococcal glucuronoxylomannan
Abstract
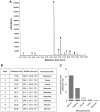
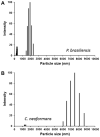
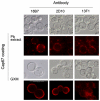
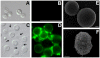
The cell wall of the yeast form of the dimorphic fungus Paracoccidioides brasiliensis is enriched with α1,3-glucans. In Cryptococcus neoformans, α1,3-glucans interact with glucuronoxylomannan (GXM), a heteropolysaccharide that is essential for fungal virulence. In this study, we investigated the occurrence of P. brasiliensis glycans sharing properties with cryptococcal GXM. Protein database searches in P. brasiliensis revealed the presence of sequences homologous to those coding for enzymes involved in the synthesis of GXM and capsular architecture in C. neoformans. In addition, monoclonal antibodies (mAbs) raised to cryptococcal GXM bound to P. brasiliensis cells. Using protocols that were previously established for extraction and analysis of C. neoformans GXM, we recovered a P. brasiliensis glycan fraction composed of mannose and galactose, in addition to small amounts of glucose, xylose and rhamnose. In comparison with the C. neoformans GXM, the P. brasiliensis glycan fraction components had smaller molecular dimensions. The P. brasiliensis components, nevertheless, reacted with different GXM-binding mAbs. Extracellular vesicle fractions of P. brasiliensis also reacted with a GXM-binding mAb, suggesting that the polysaccharide-like molecule is exported to the extracellular space in secretory vesicles. An acapsular mutant of C. neoformans incorporated molecules from the P. brasiliensis extract onto the cell wall, resulting in the formation of surface networks that resembled the cryptococcal capsule. Coating the C. neoformans acapsular mutant with the P. brasiliensis glycan fraction resulted in protection against phagocytosis by murine macrophages. These results suggest that P. brasiliensis and C. neoformans share metabolic pathways required for the synthesis of similar polysaccharides and that P. brasiliensis yeast cell walls have molecules that mimic certain aspects of C. neoformans GXM. These findings are important because they provide additional evidence for the sharing of antigenically similar components across phylogenetically distant fungal species. Since GXM has been shown to be important for the pathogenesis of C. neoformans and to elicit protective antibodies, the finding of similar molecules in P. brasiliensis raises the possibility that these glycans play similar functions in paracoccidiomycosis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Barbosa FM, et al. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006;8:493–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

