DSG3 as a biomarker for the ultrasensitive detection of occult lymph node metastasis in oral cancer using nanostructured immunoarrays
- PMID: 23010602
- PMCID: PMC3546158
- DOI: 10.1016/j.oraloncology.2012.08.001
DSG3 as a biomarker for the ultrasensitive detection of occult lymph node metastasis in oral cancer using nanostructured immunoarrays
Abstract
Objectives: The diagnosis of cervical lymph node metastasis in head and neck squamous cell carcinoma (HNSCC) patients constitutes an essential requirement for clinical staging and treatment selection. However, clinical assessment by physical examination and different imaging modalities, as well as by histological examination of routine lymph node cryosections can miss micrometastases, while false positives may lead to unnecessary elective lymph node neck resections. Here, we explored the feasibility of developing a sensitive assay system for desmoglein 3 (DSG3) as a predictive biomarker for lymph node metastasis in HNSCC.
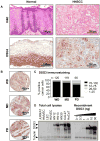
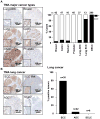
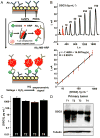
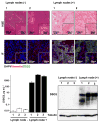
Materials and methods: DSG3 expression was determined in multiple general cancer- and HNSCC-tissue microarrays (TMAs), in negative and positive HNSCC metastatic cervical lymph nodes, and in a variety of HNSCC and control cell lines. A nanostructured immunoarray system was developed for the ultrasensitive detection of DSG3 in lymph node tissue lysates.
Results: We demonstrate that DSG3 is highly expressed in all HNSCC lesions and their metastatic cervical lymph nodes, but absent in non-invaded lymph nodes. We show that DSG3 can be rapidly detected with high sensitivity using a simple microfluidic immunoarray platform, even in human tissue sections including very few HNSCC invading cells, hence distinguishing between positive and negative lymph nodes.
Conclusion: We provide a proof of principle supporting that ultrasensitive nanostructured assay systems for DSG3 can be exploited to detect micrometastatic HNSCC lesions in lymph nodes, which can improve the diagnosis and guide in the selection of appropriate therapeutic intervention modalities for HNSCC patients.
Published by Elsevier Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–900. - PubMed
-
- Clark JR, Naranjo N, Franklin JH, de Almeida J, Gullane PJ. Established prognostic variables in N0 oral carcinoma. Otolaryngol Head Neck Surg. 2006;135(5):748–53. - PubMed
-
- Kuriakose MA, Trivedi NP. Sentinel node biopsy in head and neck squamous cell carcinoma. Current opinion in otolaryngology & head and neck surgery. 2009;17(2):100–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

