A weighted and directed interareal connectivity matrix for macaque cerebral cortex
- PMID: 23010748
- PMCID: PMC3862262
- DOI: 10.1093/cercor/bhs270
A weighted and directed interareal connectivity matrix for macaque cerebral cortex
Abstract
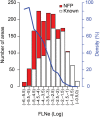
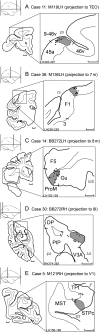
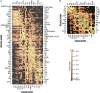
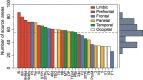
Retrograde tracer injections in 29 of the 91 areas of the macaque cerebral cortex revealed 1,615 interareal pathways, a third of which have not previously been reported. A weight index (extrinsic fraction of labeled neurons [FLNe]) was determined for each area-to-area pathway. Newly found projections were weaker on average compared with the known projections; nevertheless, the 2 sets of pathways had extensively overlapping weight distributions. Repeat injections across individuals revealed modest FLNe variability given the range of FLNe values (standard deviation <1 log unit, range 5 log units). The connectivity profile for each area conformed to a lognormal distribution, where a majority of projections are moderate or weak in strength. In the G29 × 29 interareal subgraph, two-thirds of the connections that can exist do exist. Analysis of the smallest set of areas that collects links from all 91 nodes of the G29 × 91 subgraph (dominating set analysis) confirms the dense (66%) structure of the cortical matrix. The G29 × 29 subgraph suggests an unexpectedly high incidence of unidirectional links. The directed and weighted G29 × 91 connectivity matrix for the macaque will be valuable for comparison with connectivity analyses in other species, including humans. It will also inform future modeling studies that explore the regularities of cortical networks.
Keywords: connection; cortex; graph; monkey; network.
Figures

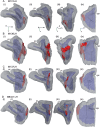
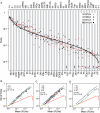
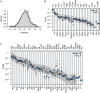
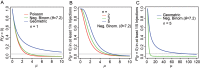



References
-
- Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 2012;22:1586–1592. - PubMed
-
- Barabasi AL, Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. - PubMed
-
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. - PubMed

