Studies of bacterial aerotaxis in a microfluidic device
- PMID: 23010909
- PMCID: PMC3520485
- DOI: 10.1039/c2lc21006a
Studies of bacterial aerotaxis in a microfluidic device
Abstract
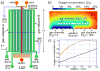
Aerotaxis, the directional motion of bacteria in gradients of oxygen, was discovered in the late 19th century and has since been reported in a variety of bacterial species. Nevertheless, quantitative studies of aerotaxis have been complicated by the lack of tools for generation of stable gradients of oxygen concentration, [O(2)]. Here we report a series of experiments on aerotaxis of Escherichia coli in a specially built experimental setup consisting of a computer-controlled gas mixer and a two-layer microfluidic device made of polydimethylsiloxane (PDMS). The setup enables generation of a variety of stable linear profiles of [O(2)] across a long gradient channel, with characteristic [O(2)] ranging from aerobic to microaerobic conditions. A suspension of E. coli cells is perfused through the gradient channel at a low speed, allowing cells enough time to explore the [O(2)] gradient, and the distribution of cells across the gradient channel is analyzed near the channel outlet at a throughput of >10(5) cells per hour. Aerotaxis experiments are performed in [O(2)] gradients with identical logarithmic slopes and varying mean concentrations, as well as in gradients with identical mean concentrations and varying slopes. Experiments in gradients with [O(2)] ranging from 0 to ~11.5% indicate that, in contrast to some previous reports, E. coli cells do not congregate at some intermediate level of [O(2)], but rather prefer the highest accessible [O(2)]. The presented technology can be applied to studies of aerotaxis of other aerobic and microaerobic bacteria.
Figures

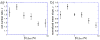
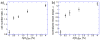
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

