Noise drives sharpening of gene expression boundaries in the zebrafish hindbrain
- PMID: 23010996
- PMCID: PMC3472692
- DOI: 10.1038/msb.2012.45
Noise drives sharpening of gene expression boundaries in the zebrafish hindbrain
Abstract
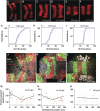
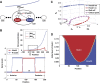
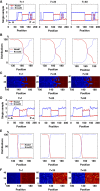
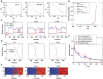
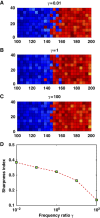
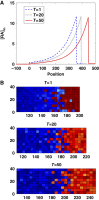
Morphogens provide positional information for spatial patterns of gene expression during development. However, stochastic effects such as local fluctuations in morphogen concentration and noise in signal transduction make it difficult for cells to respond to their positions accurately enough to generate sharp boundaries between gene expression domains. During development of rhombomeres in the zebrafish hindbrain, the morphogen retinoic acid (RA) induces expression of hoxb1a in rhombomere 4 (r4) and krox20 in r3 and r5. Fluorescent in situ hybridization reveals rough edges around these gene expression domains, in which cells co-express hoxb1a and krox20 on either side of the boundary, and these sharpen within a few hours. Computational analysis of spatial stochastic models shows, surprisingly, that noise in hoxb1a/krox20 expression actually promotes sharpening of boundaries between adjacent segments. In particular, fluctuations in RA initially induce a rough boundary that requires noise in hoxb1a/krox20 expression to sharpen. This finding suggests a novel noise attenuation mechanism that relies on intracellular noise to induce switching and coordinate cellular decisions during developmental patterning.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Alexander T, Nolte C, Krumlauf R (2009) Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol 25: 431–456 - PubMed
-
- Barrow JR, Stadler HS, Capecchi MR (2000) Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development 127: 933–944 - PubMed
-
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW (2001) The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 128: 3081–3094 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

