The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice
- PMID: 23011032
- PMCID: PMC3594029
- DOI: 10.1038/mt.2012.204
The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice
Erratum in
-
The Efficacy Versus Toxicity Profile of Combination Virotherapy and TLR Immunotherapy Highlights the Danger of Administering TLR Agonists to Oncolytic Virus-treated Mice.Mol Ther. 2013 Apr;21(4):913. doi: 10.1038/mt.2013.48. Epub 2016 Dec 6. Mol Ther. 2013. PMID: 28153327 Free PMC article. No abstract available.
Abstract
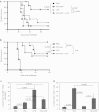
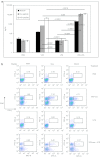
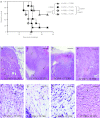
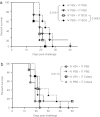
Injection of oncolytic vesicular stomatitis virus (VSV) into established B16ova melanomas results in tumor regression, in large part by inducing innate immune reactivity against the viral infection, mediated by MyD88- and type III interferon (IFN)-, but not TLR-4-, signaling. We show here that intratumoral (IT) treatment with lipopolysaccharide (LPS), a TLR-4 agonist, significantly enhanced the local therapy induced by VSV by combining activation of different innate immune pathways. Therapy was further enhanced by co-recruiting a potent antitumor, adaptive T-cell response by using a VSV engineered to express the ovalbumin tumor-associated antigen ova, in combination with LPS. However, the combination of IT LPS with systemically delivered VSV resulted in rapid morbidity and mortality in the majority of mice. Decreasing the intravenous (IV) dose of VSV to levels at which toxicity was ameliorated did not enhance therapy compared with IT LPS alone. Toxicity of the systemic VSV + IT LPS regimen was associated with rapidly elevated levels of serum tumor necrosis factor-α (TNF-α) and interleukin (IL)-6, which neither systemic VSV, nor IT LPS, alone induced. These data show that therapy associated with direct IT injections of oncolytic viruses can be significantly enhanced by combination with agonists of innate immune activation pathways, which are not themselves activated by the virus alone. Importantly, they also highlight possible, unforeseen dangers of combination therapies in which an immunotherapy, even delivered locally at the tumor site, may systemically sensitize the patient to a cytokine shock-like response triggered by IV delivery of oncolytic virus.
Figures


References
-
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N.et al. (2000Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus Nat Med 6821–825. - PubMed
-
- Colamonici OR, Domanski P, Platanias LC., and, Diaz MO. Correlation between interferon (IFN) alpha resistance and deletion of the IFN alpha/beta genes in acute leukemia cell lines suggests selection against the IFN system. Blood. 1992;80:744–749. - PubMed
-
- Grandér D., and, Einhorn S. Interferon and malignant disease–how does it work and why doesn't it always. Acta Oncol. 1998;37:331–338. - PubMed
-
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S.et al. (2003VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents Cancer Cell 4263–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

