Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2
- PMID: 23011593
- PMCID: PMC3554382
- DOI: 10.2337/db11-1116
Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2
Abstract
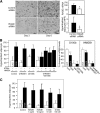
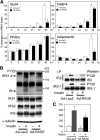
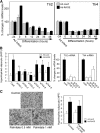
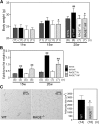
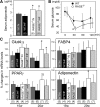
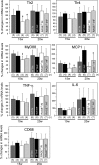
Receptor for advanced glycation end products (RAGE) has been shown to be involved in adiposity as well as atherosclerosis even in nondiabetic conditions. In this study, we examined mechanisms underlying how RAGE regulates adiposity and insulin sensitivity. RAGE overexpression in 3T3-L1 preadipocytes using adenoviral gene transfer accelerated adipocyte hypertrophy, whereas inhibitions of RAGE by small interfering RNA significantly decrease adipocyte hypertrophy. Furthermore, double knockdown of high mobility group box-1 and S100b, both of which are RAGE ligands endogenously expressed in 3T3-L1 cells, also canceled RAGE-medicated adipocyte hypertrophy, implicating a fundamental role of ligands-RAGE ligation. Adipocyte hypertrophy induced by RAGE overexpression is associated with suppression of glucose transporter type 4 and adiponectin mRNA expression, attenuated insulin-stimulated glucose uptake, and insulin-stimulated signaling. Toll-like receptor (Tlr)2 mRNA, but not Tlr4 mRNA, is rapidly upregulated by RAGE overexpression, and inhibition of Tlr2 almost completely abrogates RAGE-mediated adipocyte hypertrophy. Finally, RAGE(-/-) mice exhibited significantly less body weight, epididymal fat weight, epididymal adipocyte size, higher serum adiponectin levels, and higher insulin sensitivity than wild-type mice. RAGE deficiency is associated with early suppression of Tlr2 mRNA expression in adipose tissues. Thus, RAGE appears to be involved in mouse adipocyte hypertrophy and insulin sensitivity, whereas Tlr2 regulation may partly play a role.
Figures


References
-
- Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem 1992;267:14987–14997 - PubMed
-
- Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 1992;267:14998–15004 - PubMed
-
- Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 1999;274:31740–31749 - PubMed
-
- Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4:1025–1031 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

