TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation
- PMID: 23011795
- PMCID: PMC3478592
- DOI: 10.1073/pnas.1205742109
TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation
Abstract
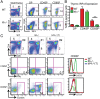
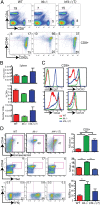
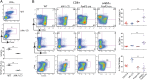
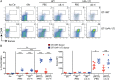
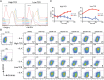
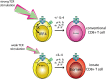
CD8(+) T-cell development in the thymus generates a predominant population of conventional naive cells, along with minor populations of "innate" T cells that resemble memory cells. Recent studies analyzing a variety of KO or knock-in mice have indicated that impairments in the T-cell receptor (TCR) signaling pathway produce increased numbers of innate CD8(+) T cells, characterized by their high expression of CD44, CD122, CXCR3, and the transcription factor, Eomesodermin (Eomes). One component of this altered development is a non-CD8(+) T cell-intrinsic role for IL-4. To determine whether reduced TCR signaling within the CD8(+) T cells might also contribute to this pathway, we investigated the role of the transcription factor, IFN regulatory factor 4 (IRF4). IRF4 is up-regulated following TCR stimulation in WT T cells; further, this up-regulation is impaired in T cells treated with a small-molecule inhibitor of the Tec family tyrosine kinase, IL-2 inducible T-cell kinase (ITK). In contrast to WT cells, activation of IRF4-deficient CD8(+) T cells leads to rapid and robust expression of Eomes, which is further enhanced by IL-4 stimulation. In addition, inhibition of ITK together with IL-4 increases Eomeso up-regulation. These data indicate that ITK signaling promotes IRF4 up-regulation following CD8(+) T-cell activation and that this signaling pathway normally suppresses Eomes expression, thereby regulating the differentiation pathway of CD8(+) T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7:479–485. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Atherly LO, et al. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. - PubMed
-
- Broussard C, et al. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. and correction (2006) 25:849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

