Catalytic, asymmetric halofunctionalization of alkenes--a critical perspective
- PMID: 23011853
- PMCID: PMC3529098
- DOI: 10.1002/anie.201204347
Catalytic, asymmetric halofunctionalization of alkenes--a critical perspective
Abstract
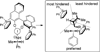
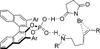
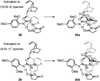
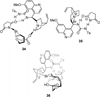
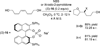
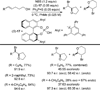
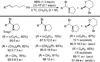
Despite the fact that halogenation of alkenes has been known for centuries, enantioselective variants of this reaction have only recently been developed. In the past three years, catalytic enantioselective versions of halofunctionalizations with the four common halogens have appeared and although important breakthroughs, they represent just the very beginnings of a nascent field. This Minireview provides a critical analysis of the challenges that accompany the development of general and highly enantioselective halofunctionalization reactions. Moreover, the focus herein, diverges from previous reviews of the field by identifying the various modes of catalysis and the different strategies implemented for asymmetric induction.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


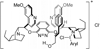
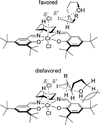




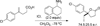
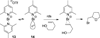

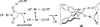


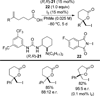
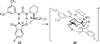
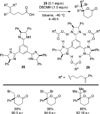
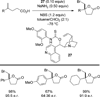
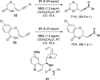



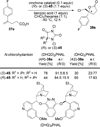
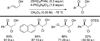

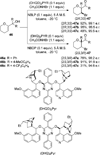
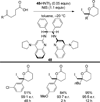

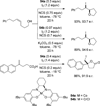
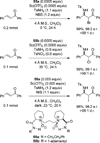

References
-
- Gilman H. Organic Chemistry: An Advanced Treatise. Vol. 1. New York: Wiley; 1938. pp. 36–43.
-
- Ingold CK. Structure and Mechanism in Organic Chemistry. Ithaca: Cornell University Press; 1953. pp. 658–670.
-
- Barton DHR. Experientia. 1950;6:316–320. - PubMed
- Fieser LF, Fieser M. Steroids. New York: Reinhold; 1959. pp. 26–41.
-
-
In passing, it is interesting to note that dibromocholesterol was already reported in 1868! Wislicenus J, Moldenhauer W. Justus Liebigs Ann. Chem. 1868;146:175–180.
-
-
- French AN, Bissmire S, Wirth T. Chem. Soc. Rev. 2004;33:354–362. - PubMed
- Bartlett PA. In: Asymmetric Synthesis. Morrison J, editor. Vol. 3. Orlando: Academic Press; 1983. pp. 411–454.
- Bartlett PA, Richardson DP, Myerson J. Tetrahedron. 1984;40:2317–2327.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

