Oxidative stress and myocardial injury in the diabetic heart
- PMID: 23011912
- PMCID: PMC3620567
- DOI: 10.1002/path.4113
Oxidative stress and myocardial injury in the diabetic heart
Abstract
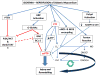
Reactive oxygen or nitrogen species play an integral role in both myocardial injury and repair. This dichotomy is differentiated at the level of species type, amount and duration of free radical generated. Homeostatic mechanisms designed to prevent free radical generation in the first instance, scavenge, or enzymatically convert them to less toxic forms and water, playing crucial roles in the maintenance of cellular structure and function. The outcome between functional recovery and dysfunction is dependent upon the inherent ability of these homeostatic antioxidant defences to withstand acute free radical generation, in the order of seconds to minutes. Alternatively, pre-existent antioxidant capacity (from intracellular and extracellular sources) may regulate the degree of free radical generation. This converts reactive oxygen and nitrogen species to the role of second messenger involved in cell signalling. The adaptive capacity of the cell is altered by the balance between death or survival signal converging at the level of the mitochondria, with distinct pathophysiological consequences that extends the period of injury from hours to days and weeks. Hyperglycaemia, hyperlipidaemia and insulin resistance enhance oxidative stress in the diabetic myocardium that cannot adapt to ischaemia-reperfusion. Altered glucose flux, mitochondrial derangements and nitric oxide synthase uncoupling in the presence of decreased antioxidant defence and impaired prosurvival cell signalling may render the diabetic myocardium more vulnerable to injury, remodelling and heart failure.
Copyright © 2012 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Figures
References
-
- Brown JR, Brown JR, Edwards FH, et al. The diabetic disadvantage: historical outcomes measures in diabetic patients undergoing cardiac surgery -- the pre-intravenous insulin era. Seminars in thoracic and cardiovascular surgery. 2006;18:281–288. - PubMed
-
- Miketic JK, Miketic JK, Hravnak M, et al. Factors influencing the outcomes of patients with both coronary artery disease and diabetes enrolled in standard cardiac rehabilitation programs: a literature review. The Journal of cardiovascular nursing. 2011;26:210–217. - PubMed
-
- Fisher BM, Fisher BM. Heart abnormalities in IDDM. Diabetologia. 1997;40 (Suppl 2):S127–129. - PubMed
-
- Flaherty JD, Flaherty JD, Davidson CJ, et al. Diabetes and coronary revascularization. JAMA : the journal of the American Medical Association. 2005;293:1501–1508. - PubMed

