GILT expression in B cells diminishes cathepsin S steady-state protein expression and activity
- PMID: 23012103
- PMCID: PMC3706190
- DOI: 10.1002/eji.201242379
GILT expression in B cells diminishes cathepsin S steady-state protein expression and activity
Abstract
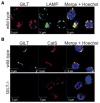
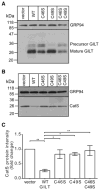
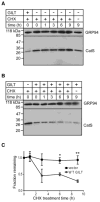
MHC class II-restricted Ag processing requires protein degradation in the endocytic pathway for the activation of CD4(+) T cells. Gamma-interferon-inducible lysosomal thiol reductase (GILT) facilitates Ag processing by reducing protein disulfide bonds in this compartment. Lysosomal cysteine protease cathepsin S (CatS) contains disulfide bonds and mediates essential steps in MHC class II-restricted processing, including proteolysis of large polypeptides and cleavage of the invariant chain. We sought to determine whether GILT's reductase activity regulates CatS expression and function. Confocal microscopy confirmed that GILT and CatS colocalized within lysosomes of B cells. GILT expression posttranscriptionally decreased the steady-state protein expression of CatS in primary B cells and B-cell lines. GILT did not substantially alter the expression of other lysosomal proteins, including H2-M, H2-O, or CatL. GILT's reductase active site was necessary for diminished CatS protein levels, and GILT expression decreased the half-life of CatS, suggesting that GILT-mediated reduction of protein disulfide bonds enhances CatS degradation. GILT expression decreased the proteolysis of a CatS selective substrate. This study illustrates a physiologic mechanism that regulates CatS and has implications for fine tuning MHC class II-restricted Ag processing and for the development of CatS inhibitors, which are under investigation for the treatment of autoimmune disease.
© 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures

Similar articles
-
γ-Interferon-inducible lysosomal thiol reductase (GILT) maintains phagosomal proteolysis in alternatively activated macrophages.J Biol Chem. 2014 Nov 14;289(46):31891-31904. doi: 10.1074/jbc.M114.584391. Epub 2014 Sep 24. J Biol Chem. 2014. PMID: 25253686 Free PMC article.
-
Cathepsin S regulates the expression of cathepsin L and the turnover of gamma-interferon-inducible lysosomal thiol reductase in B lymphocytes.J Biol Chem. 2001 Jun 22;276(25):22573-8. doi: 10.1074/jbc.M101851200. Epub 2001 Apr 16. J Biol Chem. 2001. PMID: 11306582
-
Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing.J Immunol. 2006 Dec 15;177(12):8569-77. doi: 10.4049/jimmunol.177.12.8569. J Immunol. 2006. PMID: 17142755
-
Diverse cellular and organismal functions of the lysosomal thiol reductase GILT.Mol Immunol. 2015 Dec;68(2 Pt A):124-8. doi: 10.1016/j.molimm.2015.06.008. Epub 2015 Jun 23. Mol Immunol. 2015. PMID: 26116226 Free PMC article. Review.
-
Expanding roles for GILT in immunity.Curr Opin Immunol. 2013 Feb;25(1):103-8. doi: 10.1016/j.coi.2012.11.006. Epub 2012 Dec 12. Curr Opin Immunol. 2013. PMID: 23246037 Free PMC article. Review.
Cited by
-
The Other Function: Class II-Restricted Antigen Presentation by B Cells.Front Immunol. 2017 Mar 23;8:319. doi: 10.3389/fimmu.2017.00319. eCollection 2017. Front Immunol. 2017. PMID: 28386257 Free PMC article. Review.
-
GILT restricts the cellular entry mediated by the envelope glycoproteins of SARS-CoV, Ebola virus and Lassa fever virus.Emerg Microbes Infect. 2019;8(1):1511-1523. doi: 10.1080/22221751.2019.1677446. Emerg Microbes Infect. 2019. PMID: 31631785 Free PMC article.
-
Microglia express gamma-interferon-inducible lysosomal thiol reductase in the brains of Alzheimer's disease and Nasu-Hakola disease.Intractable Rare Dis Res. 2018 Nov;7(4):251-257. doi: 10.5582/irdr.2018.01119. Intractable Rare Dis Res. 2018. PMID: 30560017 Free PMC article.
-
B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers.Front Immunol. 2020 May 8;11:760. doi: 10.3389/fimmu.2020.00760. eCollection 2020. Front Immunol. 2020. PMID: 32457742 Free PMC article. Review.
-
Conformational instability governed by disulfide bonds partitions the dominant from subdominant helper T-cell responses specific for HIV-1 envelope glycoprotein gp120.Vaccine. 2015 Jun 9;33(25):2887-96. doi: 10.1016/j.vaccine.2015.04.082. Epub 2015 May 2. Vaccine. 2015. PMID: 25944298 Free PMC article.
References
-
- Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. - PubMed
-
- Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–462. - PubMed
-
- Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

