GILT expression in B cells diminishes cathepsin S steady-state protein expression and activity
- PMID: 23012103
- PMCID: PMC3706190
- DOI: 10.1002/eji.201242379
GILT expression in B cells diminishes cathepsin S steady-state protein expression and activity
Abstract
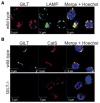
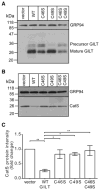
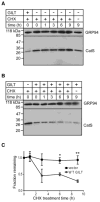
MHC class II-restricted Ag processing requires protein degradation in the endocytic pathway for the activation of CD4(+) T cells. Gamma-interferon-inducible lysosomal thiol reductase (GILT) facilitates Ag processing by reducing protein disulfide bonds in this compartment. Lysosomal cysteine protease cathepsin S (CatS) contains disulfide bonds and mediates essential steps in MHC class II-restricted processing, including proteolysis of large polypeptides and cleavage of the invariant chain. We sought to determine whether GILT's reductase activity regulates CatS expression and function. Confocal microscopy confirmed that GILT and CatS colocalized within lysosomes of B cells. GILT expression posttranscriptionally decreased the steady-state protein expression of CatS in primary B cells and B-cell lines. GILT did not substantially alter the expression of other lysosomal proteins, including H2-M, H2-O, or CatL. GILT's reductase active site was necessary for diminished CatS protein levels, and GILT expression decreased the half-life of CatS, suggesting that GILT-mediated reduction of protein disulfide bonds enhances CatS degradation. GILT expression decreased the proteolysis of a CatS selective substrate. This study illustrates a physiologic mechanism that regulates CatS and has implications for fine tuning MHC class II-restricted Ag processing and for the development of CatS inhibitors, which are under investigation for the treatment of autoimmune disease.
© 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures

References
-
- Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. - PubMed
-
- Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–462. - PubMed
-
- Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

