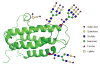
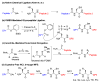
At last: erythropoietin as a single glycoform
- PMID: 23012228
- PMCID: PMC3500780
- DOI: 10.1002/anie.201206090
At last: erythropoietin as a single glycoform
Figures









References
-
- Elliott S, Foote MA, Molineux G. Erythropoietins, Erythropoietic Factors, and Erythropoiesis. 2. Birkhäuser; Basel-Boston-Berlin: 2009.
-
- Sytkowski AJ. Erythropoietin. Wiley-VCH Verlag GmbH and Co; KGaG Weinheim J: 2004.
-
- Szenajch, Wcislo G, Jeong J-Y, Szczylik C, Feldman L. Biochim Biophys Acta. 2010;1806:82. - PubMed
-
- Rush RS, Derby PL, Smith DM, Merry C, Rogers G, Rohde MF, Viswanatham K. Anal Chem. 1995;67:1442. - PubMed
-
- Dwek RA. Chem Rev. 1996;96:683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

