Apo-intermediate in the transport cycle of lactose permease (LacY)
- PMID: 23012238
- PMCID: PMC3497813
- DOI: 10.1073/pnas.1211183109
Apo-intermediate in the transport cycle of lactose permease (LacY)
Abstract
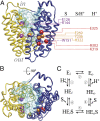
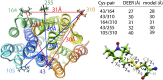
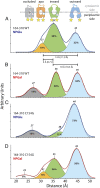
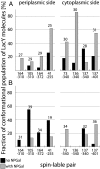
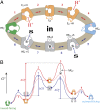
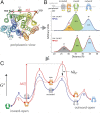
The lactose permease (LacY) catalyzes coupled stoichiometric symport of a galactoside and an H(+). Crystal structures reveal 12, mostly irregular, transmembrane α-helices surrounding a cavity with sugar- and H(+)- binding sites at the apex, which is accessible from the cytoplasm and sealed on the periplasmic side (an inward-facing conformer). An outward-facing model has also been proposed based on biochemical and spectroscopic measurements, as well as the X-ray structure of a related symporter. Converging lines of evidence demonstrate that LacY functions by an alternating access mechanism. Here, we generate a model for an apo-intermediate of LacY based on crystallographic coordinates of LacY and the oligopeptide/H(+) symporter. The model exhibits a conformation with an occluded cavity inaccessible from either side of the membrane. Furthermore, kinetic considerations and double electron-electron resonance measurements suggest that another occluded conformer with bound sugar exists during turnover. An energy profile for symport is also presented.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Engineered occluded apo-intermediate of LacY.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):12716-12721. doi: 10.1073/pnas.1816267115. Epub 2018 Nov 26. Proc Natl Acad Sci U S A. 2018. PMID: 30478058 Free PMC article.
-
The periplasmic cavity of LacY mutant Cys154→Gly: how open is open?Biochemistry. 2013 Sep 17;52(37):6568-74. doi: 10.1021/bi401026d. Epub 2013 Aug 30. Biochemistry. 2013. PMID: 23962108 Free PMC article.
-
A chemiosmotic mechanism of symport.Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1259-64. doi: 10.1073/pnas.1419325112. Epub 2015 Jan 7. Proc Natl Acad Sci U S A. 2015. PMID: 25568085 Free PMC article.
-
Lessons from lactose permease.Annu Rev Biophys Biomol Struct. 2006;35:67-91. doi: 10.1146/annurev.biophys.35.040405.102005. Annu Rev Biophys Biomol Struct. 2006. PMID: 16689628 Free PMC article. Review.
-
The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport.FEBS Lett. 2003 Nov 27;555(1):96-101. doi: 10.1016/s0014-5793(03)01087-1. FEBS Lett. 2003. PMID: 14630326 Review.
Cited by
-
Functional architecture of MFS D-glucose transporters.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):E719-27. doi: 10.1073/pnas.1400336111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550316 Free PMC article.
-
Substrate-induced changes in the structural properties of LacY.Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):E1571-80. doi: 10.1073/pnas.1404446111. Epub 2014 Apr 7. Proc Natl Acad Sci U S A. 2014. PMID: 24711390 Free PMC article.
-
Crystal structure of a eukaryotic phosphate transporter.Nature. 2013 Apr 25;496(7446):533-6. doi: 10.1038/nature12042. Epub 2013 Mar 31. Nature. 2013. PMID: 23542591 Free PMC article.
-
Substrate-binding guides individual melibiose permeases MelB to structurally soften and to destabilize cytoplasmic middle-loop C3.Structure. 2023 Jan 5;31(1):58-67.e4. doi: 10.1016/j.str.2022.11.011. Epub 2022 Dec 15. Structure. 2023. PMID: 36525976 Free PMC article.
-
Real-time conformational changes in LacY.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8440-5. doi: 10.1073/pnas.1408374111. Epub 2014 May 28. Proc Natl Acad Sci U S A. 2014. PMID: 24872451 Free PMC article.
References
-
- Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. - PubMed
-
- Saier MH, Jr, et al. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. - PubMed
-
- West IC. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970;41:655–661. - PubMed
-
- Patel L, Garcia ML, Kaback HR. Direct measurement of lactose/proton symport in Escherichia coli membrane vesicles: Further evidence for the involvement of histidine residue(s) Biochemistry. 1982;21:5805–5810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

