Identification of a karyopherin β1/β2 proline-tyrosine nuclear localization signal in huntingtin protein
- PMID: 23012356
- PMCID: PMC3501053
- DOI: 10.1074/jbc.M112.412379
Identification of a karyopherin β1/β2 proline-tyrosine nuclear localization signal in huntingtin protein
Abstract
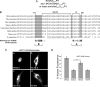
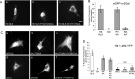
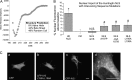
Among the known pathways of protein nuclear import, the karyopherin β2/transportin pathway is only the second to have a defined nuclear localization signal (NLS) consensus. Huntingtin, a 350-kDa protein, has defined roles in the nucleus, as well as a CRM1/exportin-dependent nuclear export signal; however, the NLS and exact pathway of import have remained elusive. Here, using a live cell assay and affinity chromatography, we show that huntingtin has a karyopherin β2-dependent proline-tyrosine (PY)-NLS in the amino terminus of the protein. This NLS comprises three consensus components: a basic charged sequence, a downstream conserved arginine, and a PY sequence. Unlike the classic PY-NLS, which has an unstructured intervening sequence between the consensus components, we show that a β sheet structured region separating the consensus elements is critical for huntingtin NLS function. The huntingtin PY-NLS is also capable of import through the importin/karyopherin β1 pathway but was not functional in all cell types tested. We propose that this huntingtin PY-NLS may comprise a new class of multiple import factor-dependent NLSs with an internal structural component that may regulate NLS activity.
Figures

References
-
- Chook Y. M., Blobel G. (2001) Karyopherins and nuclear import. Curr. Opin. Struct. Biol. 11, 703–715 - PubMed
-
- Terry L. J., Shows E. B., Wente S. R. (2007) Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 318, 1412–1416 - PubMed
-
- Cullen B. R. (2003) Nuclear mRNA export: insights from virology. Trends Biochem. Sci. 28, 419–424 - PubMed
-
- Wagstaff K. M., Jans D. A. (2009) Importins and beyond: non-conventional nuclear transport mechanisms. Traffic 10, 1188–1198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

