Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes
- PMID: 23012414
- PMCID: PMC3478642
- DOI: 10.1073/pnas.1206557109
Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes
Abstract
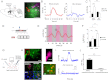
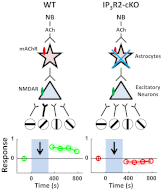
Although cholinergic innervation of the cortex by the nucleus basalis (NB) is known to modulate cortical neuronal responses and instruct cortical plasticity, little is known about the underlying cellular mechanisms. Using cell-attached recordings in vivo, we demonstrate that electrical stimulation of the NB, paired with visual stimulation, can induce significant potentiation of visual responses in excitatory neurons of the primary visual cortex in mice. We further show with in vivo two-photon calcium imaging, ex vivo calcium imaging, and whole-cell recordings that this pairing-induced potentiation is mediated by direct cholinergic activation of primary visual cortex astrocytes via muscarinic AChRs. The potentiation is absent in conditional inositol 1,4,5 trisphosphate receptor type 2 KO mice, which lack astrocyte calcium activation, and is stimulus-specific, because pairing NB stimulation with a specific visual orientation reveals a highly selective potentiation of responses to the paired orientation compared with unpaired orientations. Collectively, these findings reveal a unique and surprising role for astrocytes in NB-induced stimulus-specific plasticity in the cerebral cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kitt CA, Höhmann C, Coyle JT, Price DL. Cholinergic innervation of mouse forebrain structures. J Comp Neurol. 1994;341:117–129. - PubMed
-
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. - PubMed
-
- Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. J Neurophysiol. 2007;98:253–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

