Localized cell death focuses mechanical forces during 3D patterning in a biofilm
- PMID: 23012477
- PMCID: PMC3503208
- DOI: 10.1073/pnas.1212429109
Localized cell death focuses mechanical forces during 3D patterning in a biofilm
Abstract
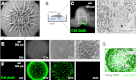
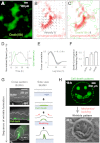
From microbial biofilm communities to multicellular organisms, 3D macroscopic structures develop through poorly understood interplay between cellular processes and mechanical forces. Investigating wrinkled biofilms of Bacillus subtilis, we discovered a pattern of localized cell death that spatially focuses mechanical forces, and thereby initiates wrinkle formation. Deletion of genes implicated in biofilm development, together with mathematical modeling, revealed that ECM production underlies the localization of cell death. Simultaneously with cell death, we quantitatively measured mechanical stiffness and movement in WT and mutant biofilms. Results suggest that localized cell death provides an outlet for lateral compressive forces, thereby promoting vertical mechanical buckling, which subsequently leads to wrinkle formation. Guided by these findings, we were able to generate artificial wrinkle patterns within biofilms. Formation of 3D structures facilitated by cell death may underlie self-organization in other developmental systems, and could enable engineering of macroscopic structures from cell populations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Turning death into creative force during biofilm engineering.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18633-4. doi: 10.1073/pnas.1215227109. Epub 2012 Nov 1. Proc Natl Acad Sci U S A. 2012. PMID: 23118336 Free PMC article. No abstract available.
References
-
- Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. - PubMed
-
- Camazine S, et al. Self-Organization in Biological Systems. Princeton Univ Press, Princeton; 2003.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

