Use of reprogrammed cells to identify therapy for respiratory papillomatosis
- PMID: 23013073
- PMCID: PMC4030597
- DOI: 10.1056/NEJMoa1203055
Use of reprogrammed cells to identify therapy for respiratory papillomatosis
Abstract
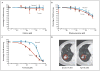
A patient with a 20-year history of recurrent respiratory papillomatosis had progressive, bilateral tumor invasion of the lung parenchyma. We used conditional reprogramming to generate cell cultures from the patient's normal and tumorous lung tissue. Analysis revealed that the laryngeal tumor cells contained a wild-type 7.9-kb human papillomavirus virus type 11 (HPV-11) genome, whereas the pulmonary tumor cells contained a 10.4-kb genome. The increased size of the latter viral genome was due to duplication of the promoter and oncogene regions. Chemosensitivity testing identified vorinostat as a potential therapeutic agent. At 3 months after treatment initiation, tumor sizes had stabilized, with durable effects at 15 months.
Figures


Comment in
-
Reprogrammed cells for respiratory papillomatosis.N Engl J Med. 2012 Dec 27;367(26):2553-4; author reply 2554. doi: 10.1056/NEJMc1212926. N Engl J Med. 2012. PMID: 23268676 No abstract available.
References
-
- Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236–47. - PubMed
-
- Donne AJ, Hampson L, Homer JJ, Hampson IN. The role of HPV type in recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2010;74:7–14. - PubMed
-
- Cook JR, Hill DA, Humphrey PA, Pfeifer JD, El-Mofty SK. Squamous cell carcinoma arising in recurrent respiratory papillomatosis with pulmonary involvement: emerging common pattern of clinical features and human papillomavirus serotype association. Mod Pathol. 2000;13:914–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
