A novel FGFR3-binding peptide inhibits FGFR3 signaling and reverses the lethal phenotype of mice mimicking human thanatophoric dysplasia
- PMID: 23014564
- PMCID: PMC3657479
- DOI: 10.1093/hmg/dds390
A novel FGFR3-binding peptide inhibits FGFR3 signaling and reverses the lethal phenotype of mice mimicking human thanatophoric dysplasia
Abstract
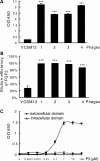
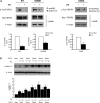
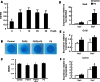
Gain-of-function mutations in fibroblast growth factor receptor-3 (FGFR3) lead to several types of human skeletal dysplasia syndromes including achondroplasia, hypochondroplasia and thanatophoric dysplasia (TD). Currently, there are no effective treatments for these skeletal dysplasia diseases. In this study, we screened, using FGFR3 as a bait, a random 12-peptide phage library and obtained 23 positive clones that share identical amino acid sequences (VSPPLTLGQLLS), named as peptide P3. This peptide had high binding specificity to the extracellular domain of FGFR3. P3 inhibited tyrosine kinase activity of FGFR3 and its typical downstream molecules, extracellular signal-regulated kinase/mitogen-activated protein kinase. P3 also promoted proliferation and chondrogenic differentiation of cultured ATDC5 chondrogenic cells. In addition, P3 alleviated the bone growth retardation in bone rudiments from mice mimicking human thanatophoric dysplasia type II (TDII). Finally, P3 reversed the neonatal lethality of TDII mice. Thus, this study identifies a novel inhibitory peptide for FGFR3 signaling, which may serve as a potential therapeutic agent for the treatment of FGFR3-related skeletal dysplasia.
Figures



References
-
- Xian C.J. Roles of epidermal growth factor family in the regulation of postnatal somatic growth. Endocr. Rev. 2007;28:284–296. - PubMed
-
- Shiang R., Thompson L.M., Zhu Y.Z., Church D.M., Fielder T.J., Bocian M., Winokur S.T., Wasmuth J.J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. - PubMed
-
- Horton W.A. Fibroblast growth factor receptor 3 and the human chondrodysplasias. Curr. Opin. Pediatr. 1997;9:437–442. - PubMed
-
- Bonaventure J., Rousseau F., Legeai-Mallet L., Le Merrer M., Munnich A., Maroteaux P. Common mutations in the fibroblast growth factor receptor 3 (FGFR 3) gene account for achondroplasia, hypochondroplasia, and thanatophoric dwarfism. Am. J. Med. Genet. 1996;63:148–154. - PubMed
-
- Rousseau F., el Ghouzzi V., Delezoide A.L., Legeai-Mallet L., Le Merrer M., Munnich A., Bonaventure J. Missense FGFR3 mutations create cysteine residues in thanatophoric dwarfism type I (TD1) Hum. Mol. Genet. 1996;5:509–512. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

