Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism
- PMID: 23015148
- PMCID: PMC3537876
- DOI: 10.1007/s00109-012-0961-5
Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism
Abstract
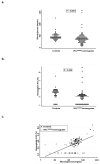
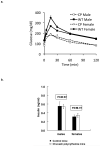
In Chuvash polycythemia, a homozygous 598C>T mutation in the von Hippel-Lindau gene (VHL) leads to an R200W substitution in VHL protein, impaired degradation of α-subunits of hypoxia-inducible factor (HIF)-1 and HIF-2, and augmented hypoxic responses during normoxia. Chronic hypoxia of high altitude is associated with decreased serum glucose and insulin concentrations. Other investigators reported that HIF-1 promotes cellular glucose uptake by increased expression of GLUT1 and increased glycolysis by increased expression of enzymes such as PDK. On the other hand, inactivation of Vhl in murine liver leads to hypoglycemia associated with a HIF-2-related decrease in the expression of the gluconeogenic enzyme genes Pepck, G6pc, and Glut2. We therefore hypothesized that glucose concentrations are decreased in individuals with Chuvash polycythemia. We found that 88 Chuvash VHL ( R200W ) homozygotes had lower random glucose and glycosylated hemoglobin A1c levels than 52 Chuvash subjects with wild-type VHL alleles. Serum metabolomics revealed higher glycerol and citrate levels in the VHL ( R200W ) homozygotes. We expanded these observations in VHL ( R200W ) homozygote mice and found that they had lower fasting glucose values and lower glucose excursions than wild-type control mice but no change in fasting insulin concentrations. Hepatic expression of Glut2 and G6pc, but not Pdk2, was decreased, and skeletal muscle expression of Glut1, Pdk1, and Pdk4 was increased. These results suggest that both decreased hepatic gluconeogenesis and increased skeletal uptake and glycolysis contribute to the decreased glucose concentrations. Further study is needed to determine whether pharmacologically manipulating HIF expression might be beneficial for treatment of diabetic patients.
Figures
Similar articles
-
Iron deficiency modifies gene expression variation induced by augmented hypoxia sensing.Blood Cells Mol Dis. 2014 Jan;52(1):35-45. doi: 10.1016/j.bcmd.2013.07.016. Epub 2013 Aug 28. Blood Cells Mol Dis. 2014. PMID: 23993337 Free PMC article.
-
Pulmonary artery pressure and iron deficiency in patients with upregulation of hypoxia sensing due to homozygous VHL(R200W) mutation (Chuvash polycythemia).Haematologica. 2012 Feb;97(2):193-200. doi: 10.3324/haematol.2011.051839. Epub 2011 Oct 11. Haematologica. 2012. PMID: 21993671 Free PMC article.
-
Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia.Am J Hum Genet. 2003 Aug;73(2):412-9. doi: 10.1086/377108. Epub 2003 Jul 3. Am J Hum Genet. 2003. PMID: 12844285 Free PMC article.
-
Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis.Blood. 2009 Sep 3;114(10):2015-9. doi: 10.1182/blood-2009-05-189985. Epub 2009 Jun 3. Blood. 2009. PMID: 19494350 Review.
-
The Role of VHL in the Development of von Hippel-Lindau Disease and Erythrocytosis.Genes (Basel). 2022 Feb 17;13(2):362. doi: 10.3390/genes13020362. Genes (Basel). 2022. PMID: 35205407 Free PMC article. Review.
Cited by
-
Hypoxia-inducible factors and diabetes.J Clin Invest. 2020 Oct 1;130(10):5063-5073. doi: 10.1172/JCI137556. J Clin Invest. 2020. PMID: 32809974 Free PMC article. Review.
-
HIF2α Is an Essential Molecular Brake for Postprandial Hepatic Glucagon Response Independent of Insulin Signaling.Cell Metab. 2016 Mar 8;23(3):505-16. doi: 10.1016/j.cmet.2016.01.004. Epub 2016 Feb 4. Cell Metab. 2016. PMID: 26853750 Free PMC article.
-
Increased transferrin protects from thrombosis in Chuvash erythrocytosis.Am J Hematol. 2023 Oct;98(10):1532-1539. doi: 10.1002/ajh.27021. Epub 2023 Jul 12. Am J Hematol. 2023. PMID: 37435906 Free PMC article.
-
Unilateral digital arterial ligation combined with low molecular weight heparins in severed finger without venous anastomosis.Exp Ther Med. 2018 Jul;16(1):342-346. doi: 10.3892/etm.2018.6174. Epub 2018 May 17. Exp Ther Med. 2018. PMID: 29896259 Free PMC article.
-
Diabetic atherosclerosis: is there a role for the hypoxia-inducible factors?Biosci Rep. 2020 Aug 28;40(8):BSR20200026. doi: 10.1042/BSR20200026. Biosci Rep. 2020. PMID: 32816039 Free PMC article. Review.
References
-
- Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. - PubMed
-
- Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. - PubMed
-
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. - PubMed
-
- Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, Butman JA, Jedlickova K, Prchal JT, Polyakova LA. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–3932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL079912-04/HL/NHLBI NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- P30 DK072437/DK/NIDDK NIH HHS/United States
- I01 BX001140/BX/BLRD VA/United States
- MO1-PR10284/PR/OCPHP CDC HHS/United States
- 2 R25-HL03679-08/HL/NHLBI NIH HHS/United States
- 1R01 DK081842/DK/NIDDK NIH HHS/United States
- 1P01CA108671-O1A2/CA/NCI NIH HHS/United States
- T32 DK091317/DK/NIDDK NIH HHS/United States
- UH1 HL003679/HL/NHLBI NIH HHS/United States
- R01 DK081842/DK/NIDDK NIH HHS/United States
- P01 CA108671/CA/NCI NIH HHS/United States
- R25 HL003679/HL/NHLBI NIH HHS/United States
- R01 HL079912/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

