Spatially coordinated kinase signaling regulates local axon degeneration
- PMID: 23015435
- PMCID: PMC6621382
- DOI: 10.1523/JNEUROSCI.2039-12.2012
Spatially coordinated kinase signaling regulates local axon degeneration
Abstract
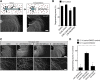
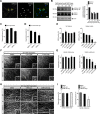
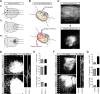
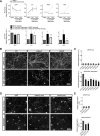
In addition to being a hallmark of neurodegenerative disease, axon degeneration is used during development of the nervous system to prune unwanted connections. In development, axon degeneration is tightly regulated both temporally and spatially. Here, we provide evidence that degeneration cues are transduced through various kinase pathways functioning in spatially distinct compartments to regulate axon degeneration. Intriguingly, glycogen synthase kinase-3 (GSK3) acts centrally, likely modulating gene expression in the cell body to regulate distally restricted axon degeneration. Through a combination of genetic and pharmacological manipulations, including the generation of an analog-sensitive kinase allele mutant mouse for GSK3β, we show that the β isoform of GSK3, not the α isoform, is essential for developmental axon pruning in vitro and in vivo. Additionally, we identify the dleu2/mir15a/16-1 cluster, previously characterized as a regulator of B-cell proliferation, and the transcription factor tbx6, as likely downstream effectors of GSK3β in axon degeneration.
Figures



References
-
- Azoulay-Alfaguter I, Yaffe Y, Licht-Murava A, Urbanska M, Jaworski J, Pietrokovski S, Hirschberg K, Eldar-Finkelman H. Distinct molecular regulation of glycogen synthase kinase-3alpha isozyme controlled by its N-terminal region: functional role in calcium/calpain signaling. J Biol Chem. 2011;286:13470–13480. - PMC - PubMed
-
- Bijur GN, Jope RS. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003;14:2415–2419. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
