Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends?
- PMID: 23015462
- PMCID: PMC3518575
- DOI: 10.1007/s12265-012-9404-5
Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends?
Abstract
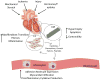
The cardiac fibroblast (CF) has historically been thought of as a quiescent cell of the heart, passively maintaining the extracellular environment for the cardiomyocytes (CM), the functional cardiac cell type. The increasingly appreciated role of the CF, however, extends well beyond matrix production, governing many aspects of cardiac function including cardiac electrophysiology and contractility. Importantly, its contributions to cardiac pathophysiology and pathologic remodeling have created a shift in the field's focus from the CM to the CF as a therapeutic target in the treatment of cardiac diseases. In response to cardiac injury, the CF undergoes a pathologic phenotypic transition into a myofibroblast, characterized by contractile smooth muscle proteins and upregulation of collagens, matrix proteins, and adhesion molecules. Further, the myofibroblast upregulates expression and secretion of a variety of pro-inflammatory, profibrotic mediators, including cytokines, chemokines, and growth factors. These mediators act in both an autocrine fashion to further activate CFs, as well as in a paracrine manner on both CMs and circulating inflammatory cells to induce myocyte dysfunction and chronic inflammation, respectively. Together, cell-specific cytokine-induced effects exacerbate pathologic remodeling and progression to HF. A better understanding of this dynamic intercellular communication will lead to novel targets for the attenuation of cardiac remodeling. Current strategies aimed at targeting cytokines have been largely unsuccessful in clinical trials, lending insights into ways that such intercellular cross talk can be more effectively attenuated. This review will summarize the current knowledge regarding CF functions in the heart and will discuss the regulation and signaling behind CF-mediated cytokine production and function. We will then highlight clinical trials that have exploited cytokine cross talk in the treatment of heart failure and provide novel strategies currently under investigation that may more effectively target pathologic CF-CM communication for the treatment of cardiac disease. This review explores novel mechanisms to directly attenuate heart failure progression through inhibition of signaling downstream of pro-inflammatory cytokines that are elevated after cardiac injury.
Figures

Similar articles
-
Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure.Cardiovasc Drugs Ther. 2020 Dec;34(6):849-863. doi: 10.1007/s10557-020-07071-0. Epub 2020 Sep 9. Cardiovasc Drugs Ther. 2020. PMID: 32902739 Free PMC article. Review.
-
Cardiac Fibrosis: The Fibroblast Awakens.Circ Res. 2016 Mar 18;118(6):1021-40. doi: 10.1161/CIRCRESAHA.115.306565. Circ Res. 2016. PMID: 26987915 Free PMC article. Review.
-
Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease.J Am Heart Assoc. 2021 Feb;10(5):e019338. doi: 10.1161/JAHA.120.019338. Epub 2021 Feb 15. J Am Heart Assoc. 2021. PMID: 33586463 Free PMC article. Review.
-
Nuclear Receptor Nur77 Controls Cardiac Fibrosis through Distinct Actions on Fibroblasts and Cardiomyocytes.Int J Mol Sci. 2021 Feb 5;22(4):1600. doi: 10.3390/ijms22041600. Int J Mol Sci. 2021. PMID: 33562500 Free PMC article.
-
The origin and arrhythmogenic potential of fibroblasts in cardiac disease.J Cardiovasc Transl Res. 2012 Dec;5(6):760-7. doi: 10.1007/s12265-012-9408-1. Epub 2012 Sep 18. J Cardiovasc Transl Res. 2012. PMID: 22987310 Free PMC article. Review.
Cited by
-
GQ262 Attenuates Pathological Cardiac Remodeling by Downregulating the Akt/mTOR Signaling Pathway.Int J Mol Sci. 2024 Sep 25;25(19):10297. doi: 10.3390/ijms251910297. Int J Mol Sci. 2024. PMID: 39408627 Free PMC article.
-
Human Adenovirus Species D Interactions with Corneal Stromal Cells.Viruses. 2021 Dec 14;13(12):2505. doi: 10.3390/v13122505. Viruses. 2021. PMID: 34960773 Free PMC article. Review.
-
Putting the Heat on Cardiac Fibrosis: Hsp20 Regulates Myocyte-To-Fibroblast Crosstalk.JACC Basic Transl Sci. 2019 Apr 29;4(2):200-203. doi: 10.1016/j.jacbts.2019.03.007. eCollection 2019 Apr. JACC Basic Transl Sci. 2019. PMID: 31061922 Free PMC article.
-
Berberine regulates proliferation, collagen synthesis and cytokine secretion of cardiac fibroblasts via AMPK-mTOR-p70S6K signaling pathway.Int J Clin Exp Pathol. 2015 Oct 1;8(10):12509-16. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26722438 Free PMC article.
-
CITED4 Protects Against Adverse Remodeling in Response to Physiological and Pathological Stress.Circ Res. 2020 Aug 14;127(5):631-646. doi: 10.1161/CIRCRESAHA.119.315881. Epub 2020 May 18. Circ Res. 2020. PMID: 32418505 Free PMC article.
References
-
- Blaxall BC, Muslin AJ. Cardiovascular therapeutic discovery. Journal of cardiovascular translational research. 2010;3:429–430. - PubMed
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:e46–e215. - PubMed
-
- Abraham WT, Greenberg BH, Yancy CW. Pharmacologic therapies across the continuum of left ventricular dysfunction. Am J Cardiol. 2008;102:21G–28G. - PubMed
-
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. - PubMed
-
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol heart failure study group. N Engl J Med. 1996;334:1349–1355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

