Challenges in O-glycan engineering of plants
- PMID: 23015807
- PMCID: PMC3449291
- DOI: 10.3389/fpls.2012.00218
Challenges in O-glycan engineering of plants
Abstract
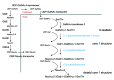
Plants are attractive alternative expression hosts for the production of recombinant proteins. Many therapeutic proteins are glycosylated with N- and O-glycosylation being the most prevalent forms of protein glycosylation. While N-glycans have already been modified in plants toward the formation of homogenous mammalian-type glycoforms with equal or improved biological function compared to mammalian-cell culture produced glycoproteins little attention has been paid to the modification of O-linked glycans. Recently, the first step of mammalian O-glycan biosynthesis has been accomplished in plants. However, as outlined in this short review there are important issues that have to be addressed in the future. These include: (i) elimination of potentially immunogenic or allergenic carbohydrate epitopes containing arabinosides or arabinogalactans, (ii) a detailed investigation of the interplay between engineered N- and O-glycosylation pathways to avoid competition for common metabolites like UDP-GlcNAc, and (iii) a deeper understanding of signals and mechanisms for distribution of glycan processing enzymes, which is a prerequisite for complete and homogenous glycosylation of recombinant proteins.
Keywords: glycoprotein therapeutics; glycosylation; metabolic engineering; molecular farming; secretory pathway.
Figures
Similar articles
-
Glycosylation of Plant-Produced Immunoglobulins.Exp Suppl. 2021;112:519-543. doi: 10.1007/978-3-030-76912-3_16. Exp Suppl. 2021. PMID: 34687021
-
A Roadmap for the Molecular Farming of Viral Glycoprotein Vaccines: Engineering Glycosylation and Glycosylation-Directed Folding.Front Plant Sci. 2020 Dec 3;11:609207. doi: 10.3389/fpls.2020.609207. eCollection 2020. Front Plant Sci. 2020. PMID: 33343609 Free PMC article. Review.
-
Engineering of sialylated mucin-type O-glycosylation in plants.J Biol Chem. 2012 Oct 19;287(43):36518-26. doi: 10.1074/jbc.M112.402685. Epub 2012 Sep 4. J Biol Chem. 2012. PMID: 22948156 Free PMC article.
-
Engineering of N. benthamiana L. plants for production of N-acetylgalactosamine-glycosylated proteins--towards development of a plant-based platform for production of protein therapeutics with mucin type O-glycosylation.BMC Biotechnol. 2010 Aug 24;10:62. doi: 10.1186/1472-6750-10-62. BMC Biotechnol. 2010. PMID: 20735851 Free PMC article.
-
Glyco-Engineering Plants to Produce Helminth Glycoproteins as Prospective Biopharmaceuticals: Recent Advances, Challenges and Future Prospects.Front Plant Sci. 2022 Apr 29;13:882835. doi: 10.3389/fpls.2022.882835. eCollection 2022. Front Plant Sci. 2022. PMID: 35574113 Free PMC article. Review.
Cited by
-
Arabinogalactan biosynthesis: Implication of AtGALT29A enzyme activity regulated by phosphorylation and co-localized enzymes for nucleotide sugar metabolism in the compartments outside of the Golgi apparatus.Plant Signal Behav. 2015;10(2):e984524. doi: 10.4161/15592324.2014.984524. Plant Signal Behav. 2015. PMID: 25723364 Free PMC article.
-
Prolyl Hydroxylase Paralogs in Nicotiana benthamiana Show High Similarity With Regard to Substrate Specificity.Front Plant Sci. 2021 Mar 2;12:636597. doi: 10.3389/fpls.2021.636597. eCollection 2021. Front Plant Sci. 2021. PMID: 33737944 Free PMC article.
-
Plant-made pharmaceuticals.Plant Biotechnol (Tokyo). 2024 Sep 25;41(3):243-260. doi: 10.5511/plantbiotechnology.24.0716a. Plant Biotechnol (Tokyo). 2024. PMID: 40177139 Free PMC article.
-
BGAL1 depletion boosts the level of β-galactosylation of N- and O-glycans in N. benthamiana.Plant Biotechnol J. 2020 Jul;18(7):1537-1549. doi: 10.1111/pbi.13316. Epub 2020 Jan 11. Plant Biotechnol J. 2020. PMID: 31837192 Free PMC article.
-
Glycosylation of Plant-Produced Immunoglobulins.Exp Suppl. 2021;112:519-543. doi: 10.1007/978-3-030-76912-3_16. Exp Suppl. 2021. PMID: 34687021
References
-
- Aggarwal S. (2010). What’s fueling the biotech engine – 2009–2010. Nat. Biotechnol. 28 1165–1171 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

