An intramolecular inverse electron demand Diels-Alder approach to annulated α-carbolines
- PMID: 23015831
- PMCID: PMC3388871
- DOI: 10.3762/bjoc.8.93
An intramolecular inverse electron demand Diels-Alder approach to annulated α-carbolines
Abstract
Intramolecular inverse electron demand cycloadditions of isatin-derived 1,2,4-triazines with acetylenic dienophiles tethered by amidations or transesterifications proceed in excellent yields to produce lactam- or lactone-fused α-carbolines. Beginning with various isatins and alkynyl dienophiles, a pilot-scale library of eighty-eight α-carbolines was prepared by using this robust methodology for biological evaluation.
Keywords: 1,2,4-triazine; chemical diversity; inverse electron demand Diels–Alder; isatin; pyrido[2,3-b]indole; α-carboline.
Figures






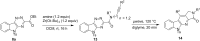


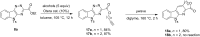
References
-
- Moquin C, Guyot M. Tetrahedron Lett. 1984;25:5047–5048. doi: 10.1016/S0040-4039(01)91115-3. - DOI
-
- Moquin-Pattey C, Guyot M. Tetrahedron. 1989;45:3445–3450. doi: 10.1016/S0040-4020(01)81023-1. - DOI
-
- Kim J-S, Shin-ya K, Furihata K, Hayakawa Y, Seto H. Tetrahedron Lett. 1997;38:3431–3434. doi: 10.1016/S0040-4039(97)00638-2. - DOI
-
- Sharaf M H M, Schiff P L, Jr, Tackie A N, Phoebe C H, Jr, Martin G E. J Heterocycl Chem. 1996;33:239–243. doi: 10.1002/jhet.5570330204. - DOI
LinkOut - more resources
Full Text Sources
