The morphology of silver nanoparticles prepared by enzyme-induced reduction
- PMID: 23016145
- PMCID: PMC3388365
- DOI: 10.3762/bjnano.3.47
The morphology of silver nanoparticles prepared by enzyme-induced reduction
Abstract
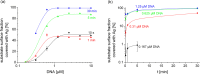
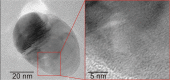
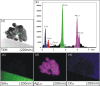
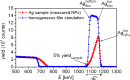
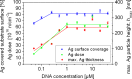
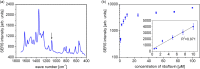
Silver nanoparticles were synthesized by an enzyme-induced growth process on solid substrates. In order to customize the enzymatically grown nanoparticles (EGNP) for analytical applications in biomolecular research, a detailed study was carried out concerning the time evolution of the formation of the silver nanoparticles, their morphology, and their chemical composition. Therefore, silver-nanoparticle films of different densities were investigated by using scanning as well as transmission electron microscopy to examine their structure. Cross sections of silver nanoparticles, prepared for analysis by transmission electron microscopy were additionally studied by energy-dispersive X-ray spectroscopy in order to probe their chemical composition. The surface coverage of substrates with silver nanoparticles and the maximum particle height were determined by Rutherford backscattering spectroscopy. Variations in the silver-nanoparticle films depending on the conditions during synthesis were observed. After an initial growth state the silver nanoparticles exhibit the so-called desert-rose or nanoflower-like structure. This complex nanoparticle structure is in clear contrast to the auto-catalytically grown spherical particles, which maintain their overall geometrical appearance while increasing their diameter. It is shown, that the desert-rose-like silver nanoparticles consist of single-crystalline plates of pure silver. The surface-enhanced Raman spectroscopic (SERS) activity of the EGNP structures is promising due to the exceptionally rough surface structure of the silver nanoparticles. SERS measurements of the vitamin riboflavin incubated on the silver nanoparticles are shown as an exemplary application for quantitative analysis.
Keywords: EGNP; SERS; enzymatically grown silver nanoparticles; enzyme-induced deposition; nanoflower.
Figures



Similar articles
-
Cubic Silver Nanoparticles Fixed on TiO2 Nanotubes as Simple and Efficient Substrates for Surface Enhanced Raman Scattering.Materials (Basel). 2019 Oct 16;12(20):3373. doi: 10.3390/ma12203373. Materials (Basel). 2019. PMID: 31623068 Free PMC article.
-
Synthesis, properties, and surface enhanced Raman scattering of gold and silver nanoparticles in chitosan matrix.J Nanosci Nanotechnol. 2009 Apr;9(4):2566-73. doi: 10.1166/jnn.2009.448. J Nanosci Nanotechnol. 2009. PMID: 19438003
-
Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities.Mater Sci Eng C Mater Biol Appl. 2016 Dec 1;69:462-9. doi: 10.1016/j.msec.2016.07.015. Epub 2016 Jul 7. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27612736
-
Biogenic Ag Nanoparticles from Neem Extract: Their Structural Evaluation and Antimicrobial Effects against Pseudomonas nitroreducens and Aspergillus unguis (NII 08123).ACS Biomater Sci Eng. 2020 Jan 13;6(1):235-245. doi: 10.1021/acsbiomaterials.9b01257. Epub 2019 Dec 11. ACS Biomater Sci Eng. 2020. PMID: 33463216
-
Surface-enhanced Raman scattering: realization of localized surface plasmon resonance using unique substrates and methods.Anal Bioanal Chem. 2009 Aug;394(7):1747-60. doi: 10.1007/s00216-009-2762-4. Epub 2009 Apr 22. Anal Bioanal Chem. 2009. PMID: 19384546 Review.
Cited by
-
Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity.Biotechnol Rep (Amst). 2014 Dec 5;5:112-119. doi: 10.1016/j.btre.2014.12.001. eCollection 2015 Mar. Biotechnol Rep (Amst). 2014. PMID: 28626689 Free PMC article.
-
High throughput electronic detection of biomarkers using enzymatically amplified metallization on nanostructured surfaces.Anal Methods. 2024 Nov 28;16(46):7854-7863. doi: 10.1039/d4ay01657b. Anal Methods. 2024. PMID: 39530206 Free PMC article.
-
Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution.Nanomaterials (Basel). 2019 Nov 2;9(11):1557. doi: 10.3390/nano9111557. Nanomaterials (Basel). 2019. PMID: 31684041 Free PMC article.
-
Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract.Asian Pac J Trop Biomed. 2013 Jan;3(1):58-63. doi: 10.1016/S2221-1691(13)60024-6. Asian Pac J Trop Biomed. 2013. PMID: 23570018 Free PMC article.
-
In Situ Investigation of the Formation Kinematics of Plasma-Generated Silver Nanoparticles.Nanomaterials (Basel). 2020 Mar 19;10(3):555. doi: 10.3390/nano10030555. Nanomaterials (Basel). 2020. PMID: 32204519 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
