Long-term efficacy and safety of atazanavir/ritonavir treatment in a real-life cohort of treatment-experienced patients with HIV type 1 infection
- PMID: 23016535
- PMCID: PMC3698683
- DOI: 10.1089/aid.2012.0092
Long-term efficacy and safety of atazanavir/ritonavir treatment in a real-life cohort of treatment-experienced patients with HIV type 1 infection
Abstract
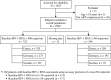
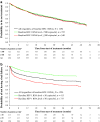
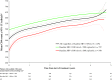
Atazanavir-based regimens have established efficacy and safety in both antiretroviral (ARV)-naive and -experienced patients. However, data evaluating effectiveness beyond 2 years is sparse. Therefore, we assessed the long-term outcomes of ritonavir-boosted atazanavir (ATV/r)-containing regimens in ARV-experienced patients in a clinical setting in a noncomparative, retrospective, observational study collecting data from three European HIV databases on ARV-experienced adults with HIV-1 infection starting an ATV/r-based regimen. Data were extracted every 6 months (maximum follow-up 5 years). Primary outcome was the proportion of patients remaining on ATV/r by baseline HIV-1 RNA (<500 or ≥500 copies/ml). Secondary outcomes included time to virologic failure, reasons for discontinuation, and long-term safety profile. The duration of treatment and time to virologic failure were analyzed using the Kaplan-Meier method. Data were analyzed for 1,294 ARV-experienced patients (male 74%; mean ART exposure 5.7 years). After 3 years, 56% (95% CI: 52%, 60%) of patients with baseline HIV-1 RNA <500 copies/ml and 53% (95% CI: 49%, 58%) of those with HIV-1 RNA ≥500 copies/ml remained on ATV/r. After 3 years, 75% (95% CI: 69%, 80%) of patients with baseline HIV-1 RNA <50 copies/ml remained suppressed and 51% (95% CI: 47%, 55%) of those with baseline HIV-1 RNA ≥50 copies/ml achieved and maintained virologic suppression. Although adverse events (AEs) were the main known reason for discontinuation, no unexpected AEs were observed. In a real-life setting ATV/r-based regimens demonstrated sustained virologic suppression in ARV-experienced patients. After long-term therapy the majority of patients remained on treatment and no unexpected AEs were observed.
Figures
References
-
- Naggie S. Hicks C. Protease inhibitor-based antiretroviral therapy in treatment-naive HIV-1-infected patients: The evidence behind the options. J Antimicrob Chemother. 2010;65:1094–1099. - PubMed
-
- Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655. - PubMed
-
- Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53:323–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

