Personalized approach of medication by indirect anticoagulants tailored to the patient-Russian context: what are the prospects?
- PMID: 23016735
- PMCID: PMC3492156
- DOI: 10.1186/1878-5085-3-10
Personalized approach of medication by indirect anticoagulants tailored to the patient-Russian context: what are the prospects?
Abstract
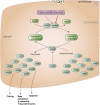
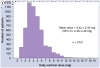
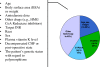
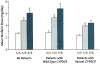
Indirect anticoagulants such as warfarin are the 'gold standard' for prevention and treatment of thromboembolic complications in patients at risk (in atrial fibrillation of valvular and nonvalvular etiology, the presence of artificial heart valves, orthopedic and trauma interventions, and other pathological conditions). A wide range of doses required to achieve a therapeutic effect indicates the need for a personalized approach to the appointment of warfarin. In addition to the dependence on the patient's clinical characteristics (sex, age, smoking status, diagnosis), there is a clear association between the warfarin dose and the carriage of certain allelic variants of key genes that makes it possible to apply molecular genetic testing for individual dose adjustment. This provides a more rapid target anticoagulant effect and also reduces the risk of bleeding associated with a possible overdose of warfarin. Implementation of this approach will allow more wide and safe application of indirect anticoagulants in Russia for needy patients.
Figures



References
-
- Moyer TP, O'Kane DJ, Baudhuin LM, Wiley CL, Fortini A, Fisher PK, Dupras DM, Chaudhry R, Thapa P, Zinsmeister AR, Heit JA. Warfarin sensitivity genotyping: a review of the literature and summary of patient experience. Mayo Clin Proc. 2009;84(12):1079–1094. doi: 10.4065/mcp.2009.0278. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

