Ionic strength effects on amyloid formation by amylin are a complicated interplay among Debye screening, ion selectivity, and Hofmeister effects
- PMID: 23016872
- PMCID: PMC3753197
- DOI: 10.1021/bi300574r
Ionic strength effects on amyloid formation by amylin are a complicated interplay among Debye screening, ion selectivity, and Hofmeister effects
Abstract
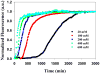
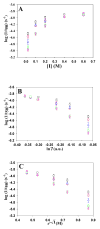
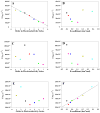
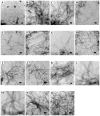
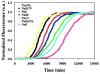
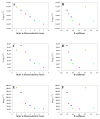
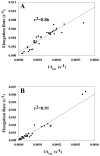
Amyloid formation plays a role in a wide range of human diseases. The rate and extent of amyloid formation depend on solution conditions, including pH and ionic strength. Amyloid fibrils often adopt structures with parallel, in-register β-sheets, which generate quasi-infinite arrays of aligned side chains. These arrangements can lead to significant electrostatic interactions between adjacent polypeptide chains. The effect of ionic strength and ion composition on the kinetics of amyloid formation by islet amyloid polypeptide (IAPP) is examined. IAPP is a basic 37-residue polypeptide responsible for islet amyloid formation in type 2 diabetes. Poisson-Boltzmann calculations revealed significant electrostatic repulsion in a model of the IAPP fibrillar state. The kinetics of IAPP amyloid formation are strongly dependent on ionic strength, varying by a factor of >10 over the range of 20-600 mM NaCl at pH 8.0, but the effect is not entirely due to Debye screening. At low ionic strengths, the rate depends strongly on the identity of the anion, varying by a factor of nearly 4, and scales with the electroselectivity series, implicating anion binding. At high ionic strengths, the rate varies by only 8% and scales with the Hofmeister series. At intermediate ionic strengths, no clear trend is detected, likely because of the convolution of different effects. The effects of salts on the growth phase and lag phase of IAPP amyloid formation are strongly correlated. At pH 5.5, where the net charge on IAPP is higher, the effect of different anions scales with the electroselectivity series at all salt concentrations.
Figures





References
-
- Abedini A, Raleigh DP. Destabilization of human IAPP amyloid fibrils by proline mutations outside of the putative amyloidogenic domain: is there a critical amyloidogenic domain in human IAPP. J Mol Biol. 2006;355:274–281. - PubMed
-
- Maurer-Stroh S, Debulpaep M, Kuemmerer N, Lopez M, Martins I, Reumers J, et al. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat Methods. 2010;7:237–242. - PubMed
-
- Moriarty DF, Raleigh DP. Effects of sequential proline substitutions on amyloid formation by human amylin 20–29. Biochemistry (Mosc) 1999;38:1811–1818. - PubMed
-
- Nerelius C, Fitzen M, Johansson J. Amino acid sequence determinants and molecular chaperones in amyloid fibril formation. Biochem Biophys Res Comm. 2010;396:2–6. - PubMed
-
- Tjernberg L, Hosia W, Bark N, Thyberg J, Johansson J. Charge attraction and beta propensity are necessary for amyloid fibril formation from tetrapeptides. J Biol Chem. 2002;277:43243–43246. - PubMed

