Safety, tolerability and pharmacokinetics of the histamine H3 receptor antagonist, ABT-288, in healthy young adults and elderly volunteers
- PMID: 23016924
- PMCID: PMC3635600
- DOI: 10.1111/j.1365-2125.2012.04472.x
Safety, tolerability and pharmacokinetics of the histamine H3 receptor antagonist, ABT-288, in healthy young adults and elderly volunteers
Abstract
Aim: The objective of this work was to characterize the safety, tolerability and pharmacokinetics of ABT-288, a highly selective histamine H3 receptor antagonist, in healthy young adults and elderly subjects following single and multiple dosing in a phase 1 setting.
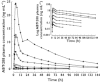
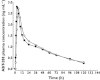
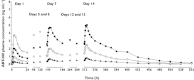
Methods: Single doses (0.1, 0.3, 1, 3, 10, 20 and 40 mg ABT-288) and multiple doses (0.5, 1.5, 3 and 6 mg ABT-288 once-daily for 14 days) were evaluated in young adults and multiple doses (0.5, 1.5, 3 and 5 mg ABT-288 once-daily for 12 days) were evaluated in elderly subjects using randomized, double-blind, placebo-controlled, dose-escalating study designs. The effect of food on ABT-288 pharmacokinetics (5 mg single dose) was evaluated using an open label, randomized, crossover design.
Results: ABT-288 safety, tolerability and pharmacokinetics were comparable in young and elderly subjects. Single doses up to 40 mg and multiple doses up to 3 mg once-daily were generally safe and well tolerated. The most frequently reported adverse events were hot flush, headache, abnormal dreams, insomnia, nausea and dizziness. ABT-288 exposure (AUC) was dose-proportional over the evaluated dose ranges. The mean elimination half-life ranged from 40 to 61 h across dose groups. Steady state was achieved by day 10 of once-daily dosing with 3.4- to 4.2-fold accumulation. Food did not have a clinically meaningful effect on ABT-288 exposure.
Conclusions: Based on the above results, 1 and 3 mg once-daily doses of ABT-288 were advanced to phase 2 evaluation in Alzheimer's patients.
© 2012 Abbott Laboratories. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures

References
-
- Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry. 2007;20:380–385. - PubMed
-
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. - PubMed
-
- Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, Lendon C, Shaw H, Bentham P. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet. 2004;363:2105–2115. - PubMed
-
- National Institute for Health and Clinical Excellence (NICE) Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease. In: NICE Technology Appraisal Guidance 217 (issue date March 2011). Available at http://www.nice.org.uk/guidance/TA217 (last accessed 30 October 2012)
-
- Leurs R, Smit MJ, Timmerman H. Molecular pharmacological aspects of histamine receptors. Pharmacol Ther. 1995;66:413–463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

