Effects of PTH on osteocyte function
- PMID: 23017659
- PMCID: PMC3552098
- DOI: 10.1016/j.bone.2012.09.016
Effects of PTH on osteocyte function
Abstract
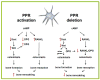
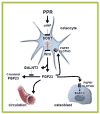
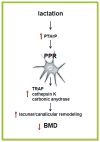
Osteocytes are ideally positioned to detect and respond to mechanical and hormonal stimuli and to coordinate the function of osteoblasts and osteoclasts. However, evidence supporting the involvement of osteocytes in specific aspects of skeletal biology has been limited mainly due to the lack of suitable experimental approaches. Few crucial advances in the field in the past several years have markedly increased our understanding of the function of osteocytes. The development of osteocytic cell lines initiated a plethora of in vitro studies that have provided insights into the unique biology of osteocytes and continue to generate novel hypotheses. Genetic approaches using promoter fragments that direct gene expression to osteocytes allowed the generation of mice with gain or loss of function of particular genes revealing their role in osteocyte function. Furthermore, evidence that Sost/sclerostin is expressed primarily in osteocytes and inhibits bone formation by osteoblasts, fueled research attempting to identify regulators of this gene as well as other osteocyte products that impact the function of osteoblasts and osteoclasts. The discovery that parathyroid hormone (PTH), a central regulator of bone homeostasis, inhibits sclerostin expression generated a cascade of studies that revealed that osteocytes are crucial target cells of the actions of PTH. This review highlights these investigations and discusses their significance for advancing our understanding of the mechanisms by which osteocytes regulate bone homeostasis and for developing therapies for bone diseases targeting osteocytes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55:287–99. - PubMed
-
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of PTH in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

