Cloning and primary characterizations of rLcn9, a new member of epididymal lipocalins in rat
- PMID: 23017836
- PMCID: PMC3459353
- DOI: 10.1093/abbs/gms072
Cloning and primary characterizations of rLcn9, a new member of epididymal lipocalins in rat
Abstract
Lipocalins are a structurally conserved and diversely functional family of proteins that are of potential importance in epididymis functions. The rat Lcn9 gene was cloned by in silico methods and genome walking based on homology to the rhesus monkey epididymal ESC513 and its polyclonal antisera were prepared. The rat Lcn9 gene is located on chromosome 3p13 spanning 7 exons, contains 2.3 kb and encodes 179 amino acids with a 17-amino acid signal peptide. Northern blot, western blot, and immunohistochemical staining analysis revealed that rat Lcn9 was a novel epididymis-specific gene, expressed selectively in the proximal caput region, influenced by luminal fluid testicular factors. Moreover, Lcn9 protein was modified by N-glycosylation and bound on the postacrosomal domain of caput sperm. In conclusion, the rat Lcn9 exhibited tissue-, region-, and temporal-specific expression patterns and its expression was regulated by luminal testicular factors. Its potential roles in sperm maturation are discussed.
Figures


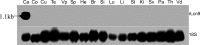
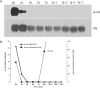




References
-
- Banks S, King SA, Irvine DS, Saunders PT. Saunders. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–514. - PubMed
-
- Dacheux JL. Protein secretion in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 151–168.
-
- Jones R. Plasma membrane composition and organisation during maturation of spermatozoa in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: from Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 405–416.
-
- Dacheux JL, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech. 2003;61:7–17. - PubMed
-
- Khole V. Epididymis as a target for contraception. Indian J Exp Biol. 2003;41:764–772. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions

