Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88
- PMID: 23018456
- PMCID: PMC3478413
- DOI: 10.4049/jimmunol.1201121
Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88
Abstract
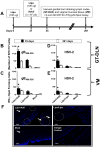
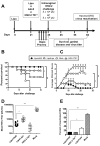
Targeting of the mucosal immune system of the genital tract with subunit vaccines has failed to induce potent and durable local CD8(+) T cell immunity, which is crucial for protection against many sexually transmitted viral pathogens, including HSV type 2 (HSV-2), which causes genital herpes. In this study, we aimed to investigate the potential of a novel lipopeptide/adenovirus type 5 (Lipo/rAdv5) prime/boost mucosal vaccine for induction of CD8(+) T cell immunity to protect the female genital tract from herpes. The lipopeptide vaccine and the rAdv5 vaccine express the immunodominant HSV-2 CD8(+) T cell epitope (gB(498-505)), and both were delivered intravaginally in the progesterone-induced B6 mouse model of genital herpes. Compared with mice immunized with the homologous lipopeptide/lipopeptide (Lipo/Lipo) vaccine, the Lipo/rAdv5 prime/boost immunized mice 1) developed potent and sustained HSV-specific CD8(+) T cells, detected in both the genital tract draining nodes and in the vaginal mucosa; 2) had significantly lower virus titers; 3) had decreased overt signs of genital herpes disease; and 4) did not succumb to lethal infection (p < 0.005) after intravaginal HSV-2 challenge. Polyfunctional CD8(+) T cells, producing IFN-γ, TNF-α, and IL-2 and exhibiting cytotoxic activity, were associated with protection (p < 0.005). The protective CD8(+) T cell response was significantly compromised in the absence of the adapter MyD88 (p = 0.0001). Taken together, these findings indicate that targeting of the vaginal mucosa with a Lipo/rAdv5 prime/boost vaccine elicits a potent, MyD88-dependent, and long-lasting mucosal CD8(+) T cell protective immunity against sexually transmitted herpes infection and disease.
Figures




References
-
- Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. - PubMed
-
- Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183:6883–6892. - PubMed
-
- King NJ, Parr EL, Parr MB. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. 1998;160:1173–1180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

