Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomised study
- PMID: 23018904
- PMCID: PMC3640146
- DOI: 10.1183/09031936.00071312
Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomised study
Abstract
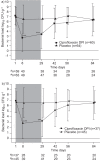
This phase II, randomised, double-blind, multicentre study (NCT00930982) investigated the safety and efficacy of ciprofloxacin dry powder for inhalation (DPI) in patients with non-cystic fibrosis bronchiectasis. Adults who were culture positive for pre-defined potential respiratory pathogens (including Pseudomonas aeruginosa and Haemophilus influenzae) were randomised to ciprofloxacin DPI 32.5 mg or placebo administered twice daily for 28 days (with 56 days of follow-up). Bacterial density in sputum (primary end-point), pulmonary function tests, health-related quality of life and safety were monitored throughout the study. 60 subjects received ciprofloxacin DPI 32.5 mg and 64 received placebo. Subjects on ciprofloxacin DPI had a significant reduction (p<0.001) in total sputum bacterial load at the end of treatment (-3.62 log10 CFU·g(-1) (range -9.78-5.02 log10 CFU·g(-1))) compared with placebo (-0.27 log10 CFU·g(-1) (range -7.96-5.25 log10 CFU·g(-1))); the counts increased thereafter. In the ciprofloxacin DPI group, 14 (35%) out of 40 subjects reported pathogen eradication at end of treatment versus four (8%) out of 49 in the placebo group (p=0.001). No abnormal safety results were reported and rates of bronchospasm were low. Ciprofloxacin DPI 32.5 mg twice daily for 28 days was well tolerated and achieved significant reductions in total bacterial load compared with placebo in subjects with non-cystic fibrosis bronchiectasis.
Keywords: Antibiotic; Pseudomonas aeruginosa; bacteria; chronic lung infection; exacerbation; inflammation.
Conflict of interest statement
Conflict of interest information can be found alongside the online version of this article at
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
