Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation
- PMID: 23019293
- PMCID: PMC3500836
- DOI: 10.1161/CIRCULATIONAHA.112.109330
Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation
Abstract
Background: Atrial fibrillation is common among older persons. Catheter ablation is increasingly used in patients for whom medical therapy has failed.
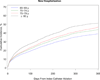
Methods and results: We conducted a retrospective cohort study of all fee-for-service Medicare beneficiaries ≥65 years of age who underwent catheter ablation for atrial fibrillation between July 1, 2007, and December 31, 2009. The main outcome measures were major complications within 30 days and mortality, heart failure, stroke, hospitalization, and repeat ablation within 1 year. A total of 15 423 patients underwent catheter ablation for atrial fibrillation. Mean age was 72 years; 41% were women; and >95% were white. For every 1000 procedures, there were 17 cases of hemopericardium requiring intervention, 8 cases of stroke, and 8 deaths within 30 days. More than 40% of patients required hospitalization within 1 year; however, atrial fibrillation or flutter was the primary discharge diagnosis in only 38.4% of cases. Eleven percent of patients underwent repeat ablation within 1 year. Renal impairment (hazard ratio, 2.07; 95% confidence interval, 1.66-2.58), age ≥80 years (hazard ratio, 3.09; 95% confidence interval, 2.32-4.11), and heart failure (hazard ratio, 2.54; 95% confidence interval, 2.07-3.13) were major risk factors for 1-year mortality. Advanced age was a major risk factor for all adverse outcomes.
Conclusions: Major complications after catheter ablation for atrial fibrillation were associated with advanced age but were fairly infrequent. Few patients underwent repeat ablation. Randomized trials are needed to inform risk-benefit calculations for older persons with drug-refractory, symptomatic atrial fibrillation.
Figures



References
-
- Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. - PubMed
-
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J. 1996;131:790–795. - PubMed
-
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. - PubMed
-
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. - PubMed
Publication types
MeSH terms
Grants and funding
- R01HL104156/HL/NHLBI NIH HHS/United States
- R21 DA027021/DA/NIDA NIH HHS/United States
- R01 HL104156/HL/NHLBI NIH HHS/United States
- RC1 HL101056/HL/NHLBI NIH HHS/United States
- R01HL068986/HL/NHLBI NIH HHS/United States
- R01 HL102214/HL/NHLBI NIH HHS/United States
- R21DA027021/DA/NIDA NIH HHS/United States
- K24 HL105780/HL/NHLBI NIH HHS/United States
- R01HL092577/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- R01 HL068986/HL/NHLBI NIH HHS/United States
- K24HL105780/HL/NHLBI NIH HHS/United States
- RC1HL101056/HL/NHLBI NIH HHS/United States
- R01HL102214/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

