Selection of antibodies that regulate phenotype from intracellular combinatorial antibody libraries
- PMID: 23019357
- PMCID: PMC3465454
- DOI: 10.1073/pnas.1214275109
Selection of antibodies that regulate phenotype from intracellular combinatorial antibody libraries
Abstract
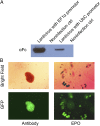
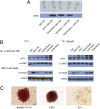
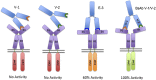
A method is presented that uses combinatorial antibody libraries to endow cells with new binding energy landscapes for the purpose of regulating their phenotypes. Antibodies that are expressed in cells infected with a lentiviral combinatorial antibody library are selected directly for function rather than only for binding. The potential diversity space can be very large because more than one lentivirus can infect a single cell. Thus, the initial combinatorial diversity of ~1.0 × 10(11) members generated by the random association of antibody heavy and light chains is greatly increased by the reassortment of the antibody Fv domains themselves inside cells. The power of the system is illustrated by its ability to select unusual antibodies. Here, the selected antibodies are potent erythropoietin agonists whose ontogeny depends on recombination at the protein level of pairs of antibodies expressed in the same cell to generate heterodimeric bispecific antibodies. The obligate synergy between the different binding specificities of the antibody's monomeric subunits appears to replicate the asymmetric binding mechanism of authentic erythropoietin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lerner RA. Manufacturing immunity to disease in a test tube: The magic bullet realized. Angew Chem Int Ed Engl. 2006;45:8106–8125. - PubMed
-
- Huse WD, et al. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989;246:1275–1281. - PubMed
-
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

