Expanding the chemical diversity of spirooxindoles via alkylative pyridine dearomatization
- PMID: 23019426
- PMCID: PMC3458780
- DOI: 10.3762/bjoc.8.111
Expanding the chemical diversity of spirooxindoles via alkylative pyridine dearomatization
Abstract
A mild and practical synthesis of spirooxindole [1,3]oxazino derivatives from N-substituted isatins and 1,3-dicarbonyl compounds with pyridine derivatives is reported. The reactions provided good to excellent yields. Further exploration of the molecular diversity of these compounds is demonstrated through Diels-Alder reactions.
Keywords: 1,3-dicarbonyl compounds; Diels–Alder reaction; chemical diversity; molecular diversity; pyridine dearomatization; spirooxindole.
Figures
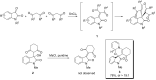
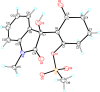
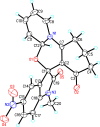

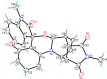
References
-
- Bindra J S. In: The Alkaloids. Manske R H F, editor. Vol. 14. New York: Academic Press; 1973. pp. 84–121.
-
- Onishi T, Sebahar P R, Williams R M. Tetrahedron. 2004;60:9503–9515. doi: 10.1016/j.tet.2004.07.047. - DOI
-
- Sebahar P R, Williams R M. J Am Chem Soc. 2000;122:5666–5667. doi: 10.1021/ja001133n. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
