Exploring chemical diversity via a modular reaction pairing strategy
- PMID: 23019462
- PMCID: PMC3458752
- DOI: 10.3762/bjoc.8.147
Exploring chemical diversity via a modular reaction pairing strategy
Abstract
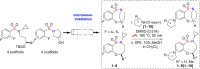
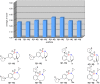
The efficient synthesis of an 80-member library of unique benzoxathiazocine 1,1-dioxides by a microwave-assisted, intermolecular nucleophilic aromatic substitution (S(N)Ar) diversification pathway is reported. Eight benzofused sultam cores were generated by means of a sulfonylation/S(N)Ar/Mitsunobu reaction pairing protocol, and subsequently diversified by intermolecular S(N)Ar with ten chiral, non-racemic amine/amino alcohol building blocks. Computational analyses were employed to explore and evaluate the chemical diversity of the library.
Keywords: benzoxathiazocine 1,1-dioxides; chemical diversity; informatics; nucleophilic aromatic substitution (SNAr); sultams.
Figures




Similar articles
-
A modular reaction pairing approach to the diversity-oriented synthesis of fused- and bridged-polycyclic sultams.Org Lett. 2011 Oct 7;13(19):5148-51. doi: 10.1021/ol201962n. Epub 2011 Sep 7. Org Lett. 2011. PMID: 21899284 Free PMC article.
-
Accessing Stereochemically Rich Sultams via Microwave-Assisted, Continuous Flow Organic Synthesis (MACOS) Scale-out.J Flow Chem. 2011 Aug;1(1):32-39. doi: 10.1556/jfchem.2011.00008. J Flow Chem. 2011. PMID: 22116791 Free PMC article.
-
Synthesis of amino-benzothiaoxazepine-1,1-dioxides utilizing a microwave-assisted, S(N)Ar protocol.ACS Comb Sci. 2011 Nov 14;13(6):653-8. doi: 10.1021/co200076j. Epub 2011 Sep 8. ACS Comb Sci. 2011. PMID: 21902243 Free PMC article.
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
-
Mitsunobu reaction: assembling C-N bonds in chiral traditional Chinese medicine.RSC Adv. 2025 Feb 17;15(7):5167-5189. doi: 10.1039/d4ra08573f. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 39963451 Free PMC article. Review.
Cited by
-
Synthesis of stereochemically and skeletally diverse fused ring systems from functionalized C-glycosides.J Org Chem. 2013 Jun 7;78(11):5160-71. doi: 10.1021/jo4000916. Epub 2013 May 21. J Org Chem. 2013. PMID: 23692141 Free PMC article.
-
Chemistry of bridged lactams and related heterocycles.Chem Rev. 2013 Aug 14;113(8):5701-65. doi: 10.1021/cr4000144. Epub 2013 Jun 17. Chem Rev. 2013. PMID: 24490625 Free PMC article. Review. No abstract available.
-
Skeletal diversification via heteroatom linkage control: preparation of bicyclic and spirocyclic scaffolds from N-substituted homopropargyl alcohols.J Org Chem. 2013 Apr 19;78(8):3720-30. doi: 10.1021/jo400077m. Epub 2013 Apr 1. J Org Chem. 2013. PMID: 23510238 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
