Organocatalytic C-H activation reactions
- PMID: 23019474
- PMCID: PMC3458764
- DOI: 10.3762/bjoc.8.159
Organocatalytic C-H activation reactions
Abstract
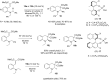
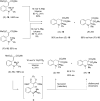
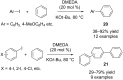
Organocatalytic C-H activation reactions have recently been developed besides the traditional metal-catalysed C-H activation reactions. The recent non-asymmetric and asymmetric C-H activation reactions mediated by organocatalysts are discussed in this review.
Keywords: C–H activation; asymmetric; non-asymmetric; organocatalysis; organocatalytic.
Figures
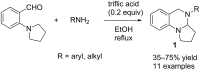

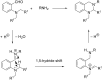
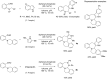
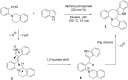
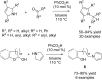
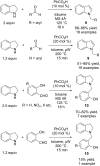

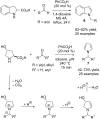
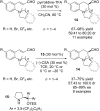




References
LinkOut - more resources
Full Text Sources
