Controlling mesenchymal stem cell gene expression using polymer-mediated delivery of siRNA
- PMID: 23020123
- PMCID: PMC3501203
- DOI: 10.1021/bm301294n
Controlling mesenchymal stem cell gene expression using polymer-mediated delivery of siRNA
Abstract
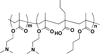
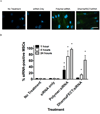
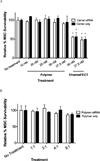
siRNA treatment has great promise to specifically control gene expression and select cell behaviors but has delivery challenges limiting its use. Particularly for applications in regenerative medicine, uniform and consistent delivery of siRNA to control gene expression and subsequent stem cell functions, such as differentiation, is paramount. Therefore, a diblock copolymer was examined for its ability to effectively deliver siRNA to mesenchymal stem cells (MSCs). The diblock copolymers, which are composed of cationic blocks for siRNA complexation, protection, and uptake and pH-responsive blocks for endosomal escape, were shown to facilitate nearly 100% MSC uptake of siRNA. This is vastly superior to a commercially available control, DharmaFECT, which resulted in only ~60% siRNA positive MSCs. Moreover, the diblock copolymer, at conditions that result in excellent knockdown (down to ~10% of control gene expression), was cytocompatible, causing no negative effects on MSC survivability. In contrast, DharmaFECT/siRNA treatment resulted in only ~60% survivability of MSCs. Longitudinal knockdown after siRNA treatment was examined and protein knockdown persists for ~6 days regardless of delivery system (diblock copolymer or DharmaFECT). Finally, MSC phenotype and differentiation capacity was examined after treatment with control siRNA. There was no statistically significant differences on cell surface markers of diblock copolymer/siRNA or DharmaFECT/siRNA-treated or cells measured 2 weeks after siRNA delivery compared to untreated cells. Upon differentiation with typical media/culture conditions to adipogenic, chondrogenic, and osteogenic lineages and examination of histological staining markers, there was no discernible differences between treated and untreated cells, regardless of delivery mechanism. Thus, diblock copolymers examined herein facilitated uniform siRNA treatment of MSCs, inducing siRNA-specific gene and protein knockdown without adversely affecting MSC survival or differentiation capacity and therefore show great promise for use within regenerative medicine applications.
Figures





Similar articles
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
The human arthritic hip joint is a source of mesenchymal stromal cells (MSCs) with extensive multipotent differentiation potential.BMC Musculoskelet Disord. 2020 May 13;21(1):297. doi: 10.1186/s12891-020-03340-z. BMC Musculoskelet Disord. 2020. PMID: 32404085 Free PMC article.
-
Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor.Stem Cells Dev. 2014 Jul 15;23(14):1594-610. doi: 10.1089/scd.2013.0477. Epub 2014 Apr 28. Stem Cells Dev. 2014. PMID: 24625206 Free PMC article.
-
Alternative splicing in mesenchymal stem cell differentiation.Stem Cells. 2020 Oct 1;38(10):1229-1240. doi: 10.1002/stem.3248. Epub 2020 Jul 15. Stem Cells. 2020. PMID: 32627865 Free PMC article. Review.
-
Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways.Tissue Eng Part B Rev. 2012 Dec;18(6):436-44. doi: 10.1089/ten.TEB.2012.0014. Epub 2012 Aug 6. Tissue Eng Part B Rev. 2012. PMID: 22741572 Free PMC article. Review.
Cited by
-
Nucleic acid delivery to mesenchymal stem cells: a review of nonviral methods and applications.J Biol Eng. 2019 Jan 18;13:7. doi: 10.1186/s13036-019-0140-0. eCollection 2019. J Biol Eng. 2019. PMID: 30675180 Free PMC article. Review.
-
Development of controlled drug delivery systems for bone fracture-targeted therapeutic delivery: A review.Eur J Pharm Biopharm. 2018 Jun;127:223-236. doi: 10.1016/j.ejpb.2018.02.023. Epub 2018 Feb 19. Eur J Pharm Biopharm. 2018. PMID: 29471078 Free PMC article. Review.
-
Diblock Copolymer Hydrophobicity Facilitates Efficient Gene Silencing and Cytocompatible Nanoparticle-Mediated siRNA Delivery to Musculoskeletal Cell Types.Biomacromolecules. 2017 Nov 13;18(11):3753-3765. doi: 10.1021/acs.biomac.7b01349. Epub 2017 Oct 11. Biomacromolecules. 2017. PMID: 28960967 Free PMC article.
-
High-throughput screening of clinically approved drugs that prime nonviral gene delivery to human Mesenchymal stem cells.J Biol Eng. 2020 May 19;14:16. doi: 10.1186/s13036-020-00238-1. eCollection 2020. J Biol Eng. 2020. PMID: 32467728 Free PMC article.
-
Evaluating side effects of nanoparticle-mediated siRNA delivery to mesenchymal stem cells using next generation sequencing and enrichment analysis.Bioeng Transl Med. 2016 Jun;1(2):193-206. doi: 10.1002/btm2.10035. Epub 2016 Oct 24. Bioeng Transl Med. 2016. PMID: 27981244 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

