Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations
- PMID: 23020132
- PMCID: PMC3549295
- DOI: 10.1056/NEJMoa1210093
Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations
Abstract
Background: Resistance to therapy with BRAF kinase inhibitors is associated with reactivation of the mitogen-activated protein kinase (MAPK) pathway. To address this problem, we conducted a phase 1 and 2 trial of combined treatment with dabrafenib, a selective BRAF inhibitor, and trametinib, a selective MAPK kinase (MEK) inhibitor.
Methods: In this open-label study involving 247 patients with metastatic melanoma and BRAF V600 mutations, we evaluated the pharmacokinetic activity and safety of oral dabrafenib (75 or 150 mg twice daily) and trametinib (1, 1.5, or 2 mg daily) in 85 patients and then randomly assigned 162 patients to receive combination therapy with dabrafenib (150 mg) plus trametinib (1 or 2 mg) or dabrafenib monotherapy. The primary end points were the incidence of cutaneous squamous-cell carcinoma, survival free of melanoma progression, and response. Secondary end points were overall survival and pharmacokinetic activity.
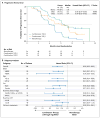
Results: Dose-limiting toxic effects were infrequently observed in patients receiving combination therapy with 150 mg of dabrafenib and 2 mg of trametinib (combination 150/2). Cutaneous squamous-cell carcinoma was seen in 7% of patients receiving combination 150/2 and in 19% receiving monotherapy (P=0.09), whereas pyrexia was more common in the combination 150/2 group than in the monotherapy group (71% vs. 26%). Median progression-free survival in the combination 150/2 group was 9.4 months, as compared with 5.8 months in the monotherapy group (hazard ratio for progression or death, 0.39; 95% confidence interval, 0.25 to 0.62; P<0.001). The rate of complete or partial response with combination 150/2 therapy was 76%, as compared with 54% with monotherapy (P=0.03).
Conclusions: Dabrafenib and trametinib were safely combined at full monotherapy doses. The rate of pyrexia was increased with combination therapy, whereas the rate of proliferative skin lesions was nonsignificantly reduced. Progression-free survival was significantly improved. (Funded by GlaxoSmithKline; ClinicalTrials.gov number, NCT01072175.).
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
