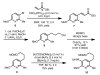
Synthesis of the pentacyclic skeleton of the indole alkaloid arboflorine
- PMID: 23020147
- PMCID: PMC3481541
- DOI: 10.1021/ol302535r
Synthesis of the pentacyclic skeleton of the indole alkaloid arboflorine
Abstract
An effective synthesis of the pentacyclic core of the unusual Kopsia alkaloid arboflorine is reported. The success of the synthetic route rested on the use of a borylative C-H functionalization reaction, a convergent Suzuki cross-coupling to a C(2) halogenated indole, and an unprecedented transannular dehydrogenative C-N bond forming reaction.
Figures
Similar articles
-
Arboflorine, an unusual pentacyclic monoterpenoid indole alkaloid incorporating a third nitrogen atom.Org Lett. 2006 Apr 13;8(8):1733-5. doi: 10.1021/ol060348k. Org Lett. 2006. PMID: 16597153
-
A photoinduced cyclization cascade-total synthesis of (-)-leuconoxine.Chemistry. 2015 Apr 20;21(17):6355-7. doi: 10.1002/chem.201500656. Epub 2015 Mar 11. Chemistry. 2015. PMID: 25761272
-
Arborisidine and Arbornamine, Two Monoterpenoid Indole Alkaloids with New Polycyclic Carbon-Nitrogen Skeletons Derived from a Common Pericine Precursor.Org Lett. 2016 Apr 1;18(7):1618-21. doi: 10.1021/acs.orglett.6b00478. Epub 2016 Mar 22. Org Lett. 2016. PMID: 27033525
-
Total Syntheses of Lycopodium and Monoterpenoid Indole Alkaloids Based on Biosynthesis-Inspired Strategies.Chem Pharm Bull (Tokyo). 2020;68(2):103-116. doi: 10.1248/cpb.c19-00872. Chem Pharm Bull (Tokyo). 2020. PMID: 32009077 Review.
-
An overview: total synthesis of arborisidine, and arbornamine.Mol Divers. 2025 Jun;29(3):2783-2797. doi: 10.1007/s11030-024-10978-7. Epub 2024 Sep 6. Mol Divers. 2025. PMID: 39242485 Review.
Cited by
-
Continuous formation of N-chloro-N,N-dialkylamine solutions in well-mixed meso-scale flow reactors.Beilstein J Org Chem. 2015 Dec 2;11:2408-17. doi: 10.3762/bjoc.11.262. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26734089 Free PMC article.
-
C-H functionalization of 2-alkyl tryptamines: direct assembly of azepino[4,5-b]indoles and total synthesis of ngouniensines.Chem Sci. 2024 Jul 5;15(32):12732-12738. doi: 10.1039/d4sc02802c. eCollection 2024 Aug 14. Chem Sci. 2024. PMID: 39148802 Free PMC article.
-
Highly Enantioselective Iridium-Catalyzed Hydrogenation of Conjugated Trisubstituted Enones.Org Lett. 2021 Jan 1;23(1):242-246. doi: 10.1021/acs.orglett.0c04012. Epub 2020 Dec 22. Org Lett. 2021. PMID: 33351634 Free PMC article.
-
Electrophilic Aminating Agents in Total Synthesis.Angew Chem Int Ed Engl. 2021 Dec 1;60(49):25640-25666. doi: 10.1002/anie.202102864. Epub 2021 Aug 6. Angew Chem Int Ed Engl. 2021. PMID: 33942955 Free PMC article. Review.
References
-
- Awang K, Svenet T, Pais M. Nat. Prod. 1993;56:1134–1139.
-
- Lim K-H, Kam T-S. Org. Lett. 2006;8:1733–1735. - PubMed
-
-
Other Kopsia indole alkaloids that possess three nitrogen atoms include the mersingines and lahadinines, see: Kam TS, Yoganathan K, Chen WJ. Nat. Prod. 1996;59:1109–1112. Kam TS, Yoganathan K. Phytochemistry. 1997;46:785–787.
-
-
-
Bioactivity including cytotoxicity toward KB and Jurkat cells and reversal of multi-drug resistance of KB cells have been noted for other alkaloids isolated from K. arborea. For example, see: Lim K-H, Hiraku O, Komiyama K, Koyano T, Hayashi M, Kam T-S. J. Nat. Prod. 2007;70:1302–1307.
-
-
- Johansen MB. Ph.D. Dissertation. The University of Western Ontario; London, ON, Canada: 2010. Part I: Synthesis of Pyrrolo[1,2-A]indoles Part II: Studies Towards Arboflorine.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous