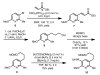
Synthesis of the pentacyclic skeleton of the indole alkaloid arboflorine
- PMID: 23020147
- PMCID: PMC3481541
- DOI: 10.1021/ol302535r
Synthesis of the pentacyclic skeleton of the indole alkaloid arboflorine
Abstract
An effective synthesis of the pentacyclic core of the unusual Kopsia alkaloid arboflorine is reported. The success of the synthetic route rested on the use of a borylative C-H functionalization reaction, a convergent Suzuki cross-coupling to a C(2) halogenated indole, and an unprecedented transannular dehydrogenative C-N bond forming reaction.
Figures
References
-
- Awang K, Svenet T, Pais M. Nat. Prod. 1993;56:1134–1139.
-
- Lim K-H, Kam T-S. Org. Lett. 2006;8:1733–1735. - PubMed
-
-
Other Kopsia indole alkaloids that possess three nitrogen atoms include the mersingines and lahadinines, see: Kam TS, Yoganathan K, Chen WJ. Nat. Prod. 1996;59:1109–1112. Kam TS, Yoganathan K. Phytochemistry. 1997;46:785–787.
-
-
-
Bioactivity including cytotoxicity toward KB and Jurkat cells and reversal of multi-drug resistance of KB cells have been noted for other alkaloids isolated from K. arborea. For example, see: Lim K-H, Hiraku O, Komiyama K, Koyano T, Hayashi M, Kam T-S. J. Nat. Prod. 2007;70:1302–1307.
-
-
- Johansen MB. Ph.D. Dissertation. The University of Western Ontario; London, ON, Canada: 2010. Part I: Synthesis of Pyrrolo[1,2-A]indoles Part II: Studies Towards Arboflorine.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous