Trastuzumab emtansine for HER2-positive advanced breast cancer
- PMID: 23020162
- PMCID: PMC5125250
- DOI: 10.1056/NEJMoa1209124
Trastuzumab emtansine for HER2-positive advanced breast cancer
Erratum in
- N Engl J Med. 2013 Jun 20;368(25):2442
Abstract
Background: Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate incorporating the human epidermal growth factor receptor 2 (HER2)-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1. The antibody and the cytotoxic agent are conjugated by means of a stable linker.
Methods: We randomly assigned patients with HER2-positive advanced breast cancer, who had previously been treated with trastuzumab and a taxane, to T-DM1 or lapatinib plus capecitabine. The primary end points were progression-free survival (as assessed by independent review), overall survival, and safety. Secondary end points included progression-free survival (investigator-assessed), the objective response rate, and the time to symptom progression. Two interim analyses of overall survival were conducted.
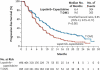
Results: Among 991 randomly assigned patients, median progression-free survival as assessed by independent review was 9.6 months with T-DM1 versus 6.4 months with lapatinib plus capecitabine (hazard ratio for progression or death from any cause, 0.65; 95% confidence interval [CI], 0.55 to 0.77; P<0.001), and median overall survival at the second interim analysis crossed the stopping boundary for efficacy (30.9 months vs. 25.1 months; hazard ratio for death from any cause, 0.68; 95% CI, 0.55 to 0.85; P<0.001). The objective response rate was higher with T-DM1 (43.6%, vs. 30.8% with lapatinib plus capecitabine; P<0.001); results for all additional secondary end points favored T-DM1. Rates of grade 3 or 4 adverse events were higher with lapatinib plus capecitabine than with T-DM1 (57% vs. 41%). The incidences of thrombocytopenia and increased serum aminotransferase levels were higher with T-DM1, whereas the incidences of diarrhea, nausea, vomiting, and palmar-plantar erythrodysesthesia were higher with lapatinib plus capecitabine.
Conclusions: T-DM1 significantly prolonged progression-free and overall survival with less toxicity than lapatinib plus capecitabine in patients with HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane. (Funded by F. Hoffmann-La Roche/Genentech; EMILIA ClinicalTrials.gov number, NCT00829166.).
Figures

Comment in
-
The promise of antibody-drug conjugates.N Engl J Med. 2012 Nov 8;367(19):1847-8. doi: 10.1056/NEJMe1211736. N Engl J Med. 2012. PMID: 23134386 No abstract available.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for meta-static breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol. 2005;23:4265–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
